Meningeal immune sentinels control neuroinflammation
Recent data indicate that the immune response in the central nervous system (CNS) is critical for proper CNS development and function, but is also linked with inflammatory and neurodegenerative diseases as well as mental health disorders. CNS immunity is thus a public health concern. However, until recently, it was believed that the CNS was devoid of most immune cell types, apart from microglia. The CNS is surrounded by layers of connective tissue, the meninges (Figure 1). Surprisingly, recent research on this understudied barrier surface revealed a great variety of immune sentinels (such as macrophages or innalte lymphocytes) that block threatening pathogens . Due to their strategic location at the interface between the periphery and the brain, the meninges function as the first line of protection of the CNS and represent a major site of immune cell recruitment. However, excessive meningeal inflammation is associated with different diseases such as autoimmune diseases, migraines or strokes, highlighting the importance of understanding the parameters of meningeal inflammation.
Meningeal innate lymphocytes foster auto-immune diseases
Multiple sclerosis is a widespread debilitating autoimmune disease with no efficient treatment available. During the disease process, autoreactive lymphocytes attack the CNS, which leads to alteration of the blood-brain barrier integrity, myelin degradation and impairment of neuronal functions. Using mouse models, we found that in the meninges that surround the CNS, inflammation can be detected even before the onset of the disease, and is the best predictor of parenchymal inflammation. Surprisingly, meningeal whole mounts revealed clusters of autoreactive CD4+ T cells and antigen-presenting cells with innate lymphoid cells (ILCs) at the surface of the CNS (Figure 2). Mechanistically, we found that meningeal ILCs promoted the expression of proinflammatory cytokines, chemokines and matrix metalloproteinases that favored T celll infiltration in the parenchyma.
As ILCs are present in the meninges but not the parenchyma, this indicates that meningeal immune responses can profoundly affect CNS pathology.
Meningeal macrophages are impaired following neuroinflammation
The CNS does not tolerate excessive inflammation. Nevertheless, neuroinvasive pathogens must be quickly controlled before they spread into the CNS. This requires efficient detection by local sentinels and rapid recruitment of peripheral immune cells. Immune infiltration through the highly restricted blood-brain barrier in the CNS parenchyma has been studied extensively but is not the only path into the CNS. Indeed, we show the existence of a vast network of immune sentinels at the surface of the brain, and much less is known about pathogen detection and immune recruitment occurring in this CNS barrier tissue. The meninges of the CNS are indeed populated by a dense network of specialized macrophages that act as imune sentinels and constantly scan the surface of the CNS (Figure 3, Video 1). Their function in the steady state and upon inflammatory conditions is unclear. We discovered that, following lymphocytic choriomeningitis virus infection, resident meningeal macrophages (MMs) were infected and killed by infiltrating cytotoxic T lymphocytes, which led to macrophage depletion (Video 2). Concurrently, the meninges were infiltrated by inflammatory monocytes . Surprisingly, those inflammatory cells engrafted the meningeal niche and remained in situ for months after viral clearance. This engraftment led to interferon-γ-dependent functional changes in the pool of MMs, including loss of bacterial and immunoregulatory sensors. Overall, our findings indicate that peripheral monocytes can engraft the meninges after an inflammatory challenge, imprinting the compartment with long-term defects in immune function.
We are thus developing new strategies to control neuroinflammation and neuroinvasion by targeting meningeal immune sentinels such as macrophages. Because they represent the first responders located at the brain surface, understanding their mechanism of action is crucial if we want to finely tune the kinetics and amplitude of CNS immune infiltration. This information is also important for the development of therapeutics to promote microbial clearance without causing deleterious neuroinflammation.
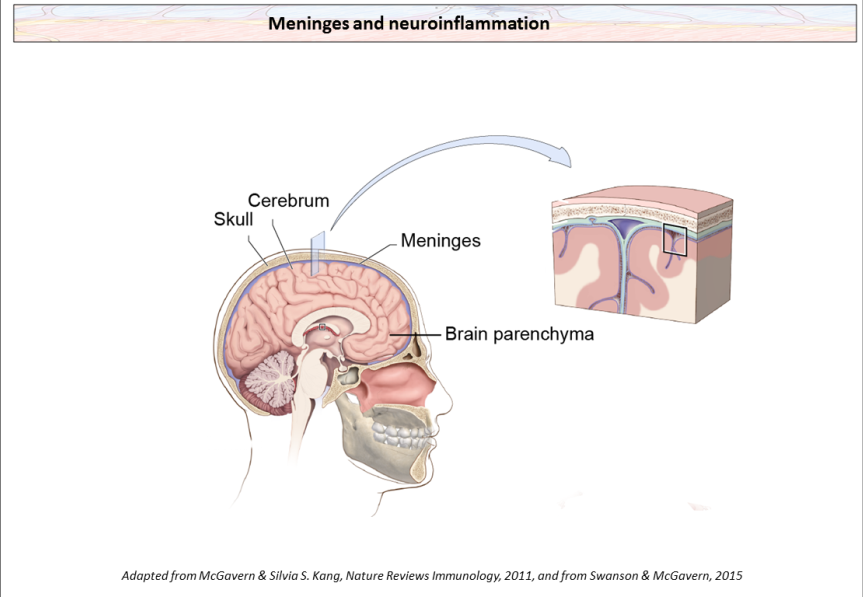
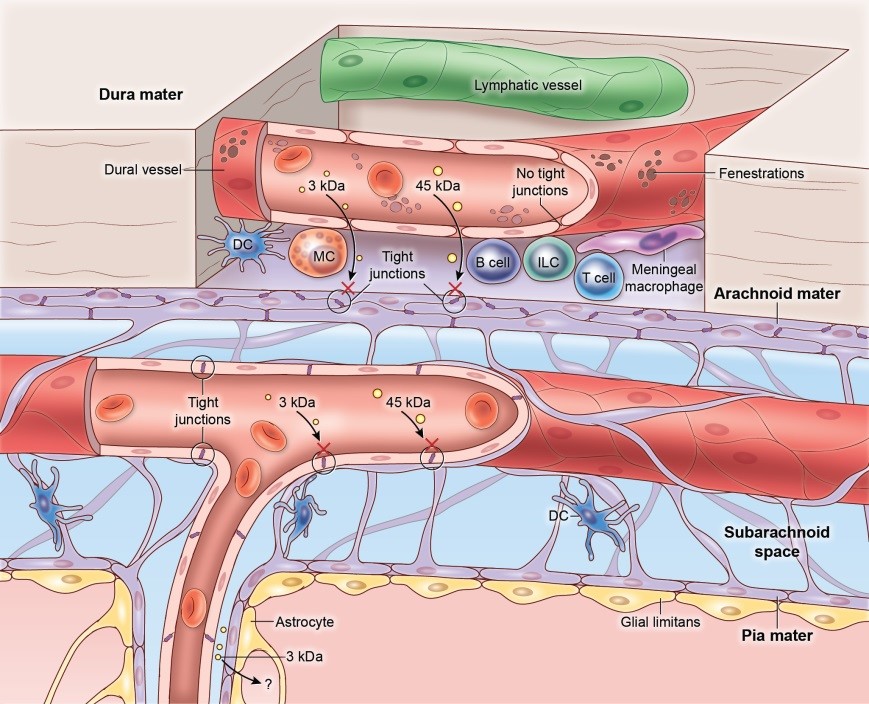
Location of the meninges(containing the dura mater, the arachnoid mater and the pia mater) at the surface of the brain. Meninges at the CNS surface are highly vascularized and are populated by macrophages, Dendritic Cells (DC), Mast cells (MC), B cells, Innate Lymphocytes (ILCs) and T cells, that are mostly absent in the brain parenchyma
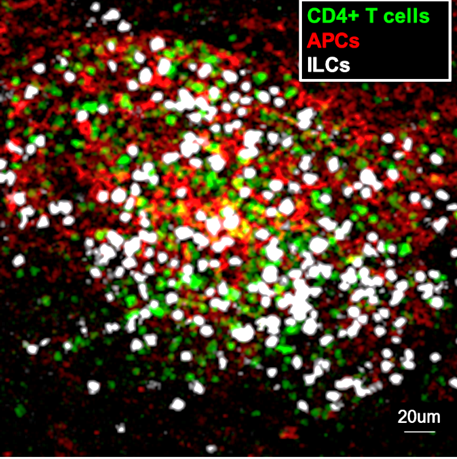
Meningeal whole mount showing an inflammatory cluster, containing auto-reactive CD4+ T cells, antigen-presenting cells (APCs) and innate lymphocytes (ILCs) at the peak of the disease
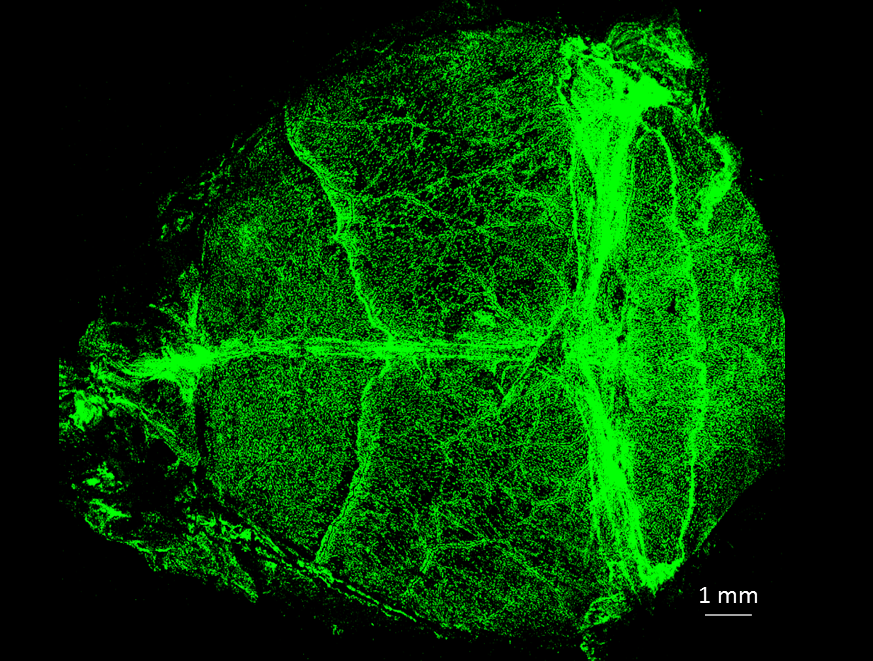
Bone-in meningeal whole mounts showing the vast network of meningeal macrophages (identified by the mannose receptor CD206) covering the brain surface.
Video 1. Intravital movie of CX3CR1-GFP mouse showing a top-down view of meningeal macrophages (green) along the vasculature (red) observed through the thinned skull under steady-state.
Video 2. Intravital movie of CX3CR1-GFP mouse at the peak of meningitis showing infiltrating T cells (pink) interacting with macrophages (green) along the vasculature (red).