Different pathogens have evolved distinct strategies to control their intracellular fate and enhance their survival within host cells. Bacteria, such as Salmonella, Mycobacterium and Brucella remain within a membrane-bound vacuole displaying various characteristics. For instance, Salmonella-containing vacuoles are characterized by the presence of multiple markers of late endocytic compartments whereas Brucella escapes the endocytic pathway and interacts with the endoplasmic reticulum.
Our aim is to understand how intracellular pathogenic bacteria reach their replication niche and what are the bacterial molecules, which play a role in the control of the immune response. During the past ten years, we have been interested in characterizing the host-pathogen interaction mechanisms that allow the bacteria to establish a long lasting life inside the host. Both in vitro and in vivo approaches have been used to study the fate of alive Salmonella, Mycobacterium and Brucella and their products in host cells, especially in macrophages and dendritic cells.
Overview
Our research team aims at studying interactions between host cells and pathogenic bacteria such as Salmonella, Brucella and Mycobacterium.
We characterized in vivo the fist target cells in the intestine and the lung after infection with Brucella and Salmonella. In the Salmonella project we have shown that the various steps involved in the maturation of the Salmonella-containing vacuole and the establishment of the replication niche are dependent of the expression of several bacterial effector proteins being secreted by the type III secretion system.
PipB2, SopD2 and SifA are three Salmonella effector proteins controlling the recruitment of kinesin-1 at the Salmonella-containing vacuole in the presence of the host protein SKIP. PipB2 plays the role of a linker for kinesin and SKIP controls membrane exchanges dependent of kinesin.
To corroborate these studies, crystal structure of the SKIP/SifA complex has been performed. In the Brucella project, we established that the endoplasmic reticulum is the replication site Brucella in dendritic cells. Our efforts have concentrated on the detection of Brucella by the immune system. We have shown that the Brucella LPS plays the role of a shield anti-recognition by the TLR-4/MD2 complex.
Also, we were the first to demonstrate that a new class of bacterial molecules called Btp for Brucella Toll-like interacting proteins, control the signalisation pathway activated by the TLR-agonist interactions.
In addition, we have identified a new virulence factor, the beta-cyclic glucan from Brucella and found that, when purified, can be used as an adjuvant. In Mycobacterium, the tight interaction between the membrane of the phagosome and the bacterium is involved in the arrest of the Mycobacterium-containing vacuole. We have shown that cholesterol is essential to maintaining this interaction. In granulomas, bacteria have the tendency to enter into a latent dormant phase.
We have shown that Mycobacterium tuberculosis induces the differentiation of phagocytes into foamy macrophages. In this type of macrophages, bacteria accumulate lipid inclusions in their cytoplasm, which serve as an energy storage compartment.
Brucella replicates and controls innate immunity within dendritic cells.
We have recently shown, using a murine intestinal loop model of infection, that intestinal DCs from the region underlying the follicle associated epithelium of Peyer’s patches were infected by B. abortus soon after inoculation.
To better evaluate the consequences of B. abortus infection on DC function, we have established murine bone marrow-derived dendritic cells as an infection model system and show that B. abortus replicates within DCs and inhibits their process of maturation, ultimately leading to a reduction in cytokine secretion and antigen presentation.
We have also identified a Brucella protein, Btp1, which contributes to inhibit maturation of infected DCs in vitro by targeting mainly the TLR2 signaling pathway, highlighting a new mechanism used by Brucella to control the immune response of the host. Infection of DCs by Brucella maintains the cells in an intermediate maturation stage, which has been shown in other circumstances to be able to promote tolerance rather than immunity against specific antigens.
Therefore, it is possible that Btp1 contributes to the establishment of chronic brucellosis by inducing tolerizing DCs in the host.
Alternatively, the migration properties of DCs could facilitate infection spreading, as suggested by the rapid interaction of DCs with penetrating Brucella from the gut.
Control of the level of DC activation during Brucella infection may provide a new strategy for more efficient vaccine design against this pathogen (Salcedo et al., PLoS pathogens, 2008).
A main role in Brucella ability to escape detection by innate immunity is played by the Brucella lipopolysaccharide (LPS), a surface molecule that confers resistance to killing by bactericidal peptides and that is barely recognized by TLR4-MD2, a key receptor system of the cytokine cascade.
Comparative studies with other bacteria and with LPS mutants have proven that the lipid A and O-chains sections of Brucella LPS are relevant in these properties. However, the role in virulence of the LPS core oligosaccharide has not been directly determined because all core mutants described so far are simultaneously devoid of the O-chain, thus making difficult to interpret the overall phenotype.
We established a B. abortus mutant (BABΔlpcC) in an ORF homologue to R. leguminosarum lpcC, the gene that encodes the transferase incorporating mannose to Kdo I (the core sugar directly linked to lipid A). The LPS of BABΔlpcC lacked part of the core but kept an intact O-chain. As compared to wild type B. abortus, BABΔlpcC was sensitive to bactericidal peptides and, although not attenuated in macrophages, it was unable to replicate in DCs, inducing their maturation and much higher levels of TNF-α and IL-12. These effects were reproduced by the LPS purified from BABΔlpcC but not by the wild type LPS and, in keeping with the cytokine induction ability, the LPS of BABΔlpcC showed a higher binding to TLR4/MD2. Consistent with the above-summarized results, BABΔlpcC was attenuated in mice and triggered a better and more sustained protective immunoresponse than that obtained with B. abortus S19, the best animal vaccine currently available.
These results demonstrate that the core and not only the O-chain of Brucella LPS is required for virulence and strongly suggest that the appropriate core mutants can improve current brucellosis animal vaccines.
In contrast to LPS moities or Btp proteins, which are able to down regulate innate immunity functions, other Brucella molecules such as the beta cyclic glucan (CβG) displays activating properties. In 2005, we showed that this sugar, composed of 20-25 glucose units facilitates the intracellular trafficking of Brucella during the first steps of invasion. We purified the molecule and showed that it constitutes a new class of non-toxic adjuvants.
We have investigated the effect of Brucella CβG and other cyclic glucans on DCs maturation, in term of production of cytokines, surface expression of MHC-II and co-stimulatory molecules, gene expression, toxicity and antibody responses on both human and mouse DCs. Our results for the first time demonstrate that Brucella CβG is a potent activator of mouse and human DCs. Furthermore, cyclic glucans with different structures did not induce the same activation responses, showing that CβG with different structures may have different activation properties.
We also show that Brucella CβG seems to be recognized by the TLR4 pathway and that MyD88 and TRIF molecules are involved in the DC maturation process.
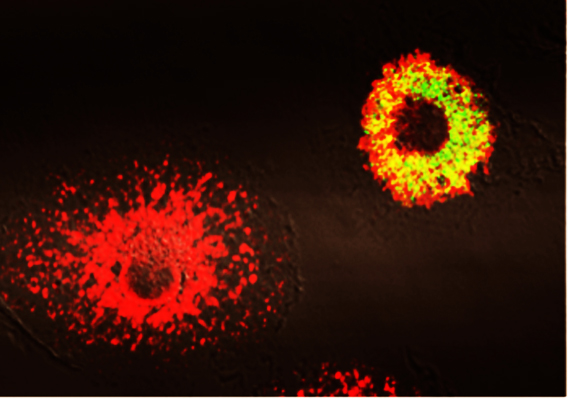 Phagocytes infected with Brucella abortus expressing the GFP (in green) at 48h post-infection. Calreticulin (in red) is a marker for the endoplamic reticulum. At the bottom left, a non-infected cell displaying a typical endoplasmic reticulum and on the right, an infected cell showing that all the endoplasmic reticulum constitutes the replication niche (appearing in yellow). Copyright JP Gorvel, CIML.
Phagocytes infected with Brucella abortus expressing the GFP (in green) at 48h post-infection. Calreticulin (in red) is a marker for the endoplamic reticulum. At the bottom left, a non-infected cell displaying a typical endoplasmic reticulum and on the right, an infected cell showing that all the endoplasmic reticulum constitutes the replication niche (appearing in yellow). Copyright JP Gorvel, CIML.
Molecular and Cellular basis of Salmonella Virulence
Salmonella is a facultative intracellular pathogen associated with a variety of diseases in humans, including typhoid fever, gastroenteritis and blood infection in immuno-compromised individuals.
The goal of this project was to understand the molecular mechanisms of Salmonella survival in the apparently unaccommodating environment of a host cell vacuole from the perspective of both pathogen and host. More specifically we tried to understand the function of late Salmonella effector proteins that are translocated from the bacteria into the host by a type 3 secretion system (T3SS-2) and which subvert many aspects of the physiology and immunity of the infected host cell, thereby promoting bacterial virulence. We focused on the function of the Salmonella T3SS-2 effectors PipB2, SifA and SopD2, which recruit kinesin-1 on the vacuole and regulate its activity.
We were able to identify the T3SS-2 effector PipB2 as a kinesin-1 linker and to demonstrate that the T3SS-2 effectors SseF and SseG form a functional complex. We also performed crystallographic and funtional analysis of the SifA-SKIP complex and identify SKIP as a regulator of kinesin-1-dependent membrane exchanges. Finally, we demonstrate that SopD2 controls most of phenotypes associated with the absence of sifA.
Survival strategies of pathogenic mycobacteria
The pathogenicity of mycobacteria (M. avium, M. tuberculosis) correlates with their ability to survive and multiply in macrophages of the host organism. It is well known that these mycobacteria prevent phagosomes from maturing and, as a result, from fusing with lysosomes. C. De Chastellier has shown that, whatever the molecular mechanisms involved in this maturation block, the establishment and maintenance of a close apposition of the phagosome membrane with the entire mycobacterial surface is a necessary requirement for prevention of phagosome maturation. Interference with the close apposition systematically leads to phagosome maturation and fusion with lysosomes.
Mycobacteria are, however, not degraded in this cytolytic environment. Instead they rescue themselves from phagolysosomes to again reside in non-matured phagosomes that have re-established a closely apposed membrane all around and that no longer fuse with lysosomes. After escape from the phagolysosome, bacteria resume growth and septation.
We have shown that the establishment and maintenance of the close apposition requires sufficient levels of cholesterol and involves modifications of phagosome membrane glycoproteins linked to cholesterol binding and transport.
The challenges faced by M. tuberculosis change dramatically as tuberculosis (TB) progresses towards confinement of bacteria within host granulomas in which bacilli can persist for decades in a phase of dormancy until favourable conditions lead to TB re-activation.
We showed by electron microscopy approaches that M. tb induces the rapid differentiation of phagocytes into foamy macrophages (FM). In FMs, the bacilli, which are all enclosed in phagosomes, are not killed but survive in a non-replicating state. M. tb-containing phagosomes interact with the FM lipid granules that they seem to engulf or fuse with. As a result, bacilli are freed into a lipid environment from which they accumulate their own intracytoplasmic lipid inclusions as is typical of “dormant” bacilli. FMs from TB patient granulomas, therefore, constitute a nutrient-rich reservoir for M.tb persistence.
Work in progress with an FM model showed that mycobacteria accumulate large amounts of lipids. Although bacteria continue to replicate DNA and other constituents, septation is blocked in this lipid-rich environment until depletion of cellular lipids.
LysoDC: a novel intestinal DC subset that expresses lysozyme and internalizes pathogenic bacteria and dead cells
Peyer’s patches (PP) of the mammal small intestine are important sites of antigen sampling and immune response initiation. They are formed by clustered B-cell follicles separated from the overlying epithelium by sub-epithelial domes (SED). The follicle-associated epithelium (FAE) contains specialized epithelial cells, called M cells, which bind and rapidly transport antigens from the lumen to the SED. The SED is enriched in DC, professional antigen-presenting cells with the unique capacity to initiate primary immune responses.
We identified a novel myeloid DC subset specifically localized in the SED of human, rat and mouse PP and absent from conventional villi. This DC subset is distinguishable from others by its high expression of the bactericidal agent lysozyme and thus hereafter referred to as LysoDC. This is the first time that a marker allows the specific detection of an intestinal DC subset mainly present in the SED.
LysoDC subset display the highest surface expression level of molecules required for antigen presentation (MHC-II, CD40, CD80, CD86), indicating they may be one of the most efficient DC subset to present antigens and initiate an immune response. They are also the main DC subset involved in the uptake of the pathogenic bacteria Salmonella Typhimurium after oral infection. In addition, they engulf dead cells, including M cells.
We also studied the dynamics of LysoDC in vivo. Importantly, based on their dynamic characteristics two subpopulations could be characterized: the former constitutes the major part of the cells forming this dense network in the SED and is mostly static thus probably representing a pool of lysozyme-secreting cells participating to the innate defence of this tissue frequently exposed to pathogens; the latter is formed by a reduced number of highly dynamic cells sending dendrites in the FAE and moving fast between other cells of the SED thus probably representing the DC pool responsible for uptake and efficient presentation of antigens.
Importantly, LysoDC extensions into the FAE are specifically associated with M-cells and could in fact reach the lumen through transcellular pores and capture microparticles and bacteria.