B cells constitute essential elements of our immune system as they provide protection from pathogenic threats. The initial recognition of pathogens by B cells takes place in secondary lymphoid organs, such as the spleen and lymph nodes. Following pathogen encounter, B cells can enter into germinal center reactions, where they undergo sequential rounds of diversification and selection, resulting in the production of antibodies with high-affinity to the invading pathogen.
By using a wide range of techniques, from two-photon microscopy and multiparametric flow cytometry to mouse genetics and in vivo infection models, we study how the lymph node microenvironment controls the magnitude of antibody responses.
Overview
B cells are key elements of adaptive immunity because of their ability to produce highly specific antibodies in response to infection. Antibodies not only confer protection from pathogenic threats, but also are the basis for most currently licensed human vaccines. Despite their great importance for public health, the precise events driving the initiation of antibody production are still unknown. Therefore, a deeper understanding of the cellular and molecular mechanisms of B cell activation in response to infection is critical in the development of next-generation vaccines. Our research focuses on the understanding of how cells from the innate branch of the immune system control the initiation of B cell immunity in secondary lymphoid organs.
Macrophages: breaking ranks in the lymph node
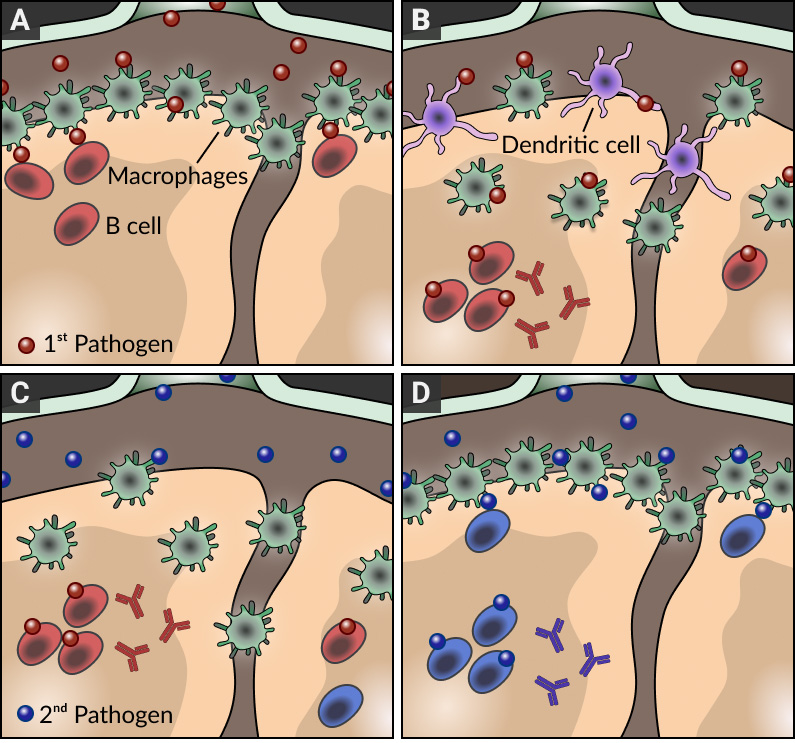
The architecture of lymph nodes is highly organized to ensure that effective immune responses develop against pathogens. In particular, the macrophages that line the subcapsular sinus of lymph nodes are strategically placed to prevent the systemic dissemination of pathogens and support antibody responses by delivering intact antigen to B cells in subjacent follicles (see panel A). By combining a number of innovative imaging approaches with the generation of a new series of transgenic mouse strains and infection models, we found that these resident macrophages do not stay in the subcapsular sinus area indefinitely; during inflammation and infection, they are displaced towards the inner follicular regions of the lymph node by migratory dendritic cells arriving from the sites of infection (panel B). While this initial disruption of the macrophage layer may enhance the response to the primary infection, this is at the cost of leaving the lymph node temporarily 'shut down' and refractory to subsequent pathogens (panel C). Importantly, this transient inability to respond to further antigenic challenge culminates once the macrophage layer is restored (panel D). Therefore, our findings not only provide a novel explanation for the temporary failure of host defenses to counteract secondary infections but also highlight the importance of timing and adequate selection of injection sites at the time of vaccination.
NKT cells: bringing help at the borders
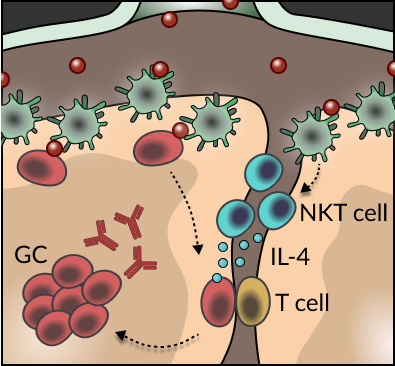
The generation of protective antibodies upon pathogen encounter requires sequential rounds of diversification of antigen-specific B cells and selection of those cells binding with high affinity to the infectious agents. These processes take place in specialized follicular structures known as germinal centers. Despite the importance of these processes in the production of high-affinity antibodies, how B cells initially seed germinal center reactions remained largely unexplored. Through the de novo development of conditional transgenic animals, the use of single-cell RNA-seq, and advanced tissue immunofluorescence, we found that an innate-like population of T cells, known as Natural Killer T (NKT) cells, are critical for the induction of B cell immunity upon viral infection. This unexpected phenomenon requires the spatial positioning of NKT cells at the interfollicular areas of lymph nodes, which facilitates both, their direct priming by resident macrophages and the localized delivery of IL-4 to antigen-specific B cells (see Scheme). Our findings not only unveil a new layer of innate regulation of B cell immunity at the early stages of infection, but also raise the prospect of harnessing the interplay between B cells and NKT cells/IL-4 in the design of future vaccination strategies.
Our goal is to better understand how the lymph node microenvironment controls the initiation of B cell immunity upon infection. To address this important question, we will take advantage of live imaging, multiparametric flow cytometry, in vivo infection models and the generation of new mouse systems.