IMMUNE TOLERANCE AND T CELL DIFFERENTIATION
Immune tolerance critically prevents potentially harmful immune responses against the host. A breakdown in tolerance can lead to many autoimmune diseases. The thymus plays a key role in the induction of T-cell tolerance either by the deletion of potentially hazardous autoreactive T cells or by the induction of regulatory T cells, which have the unique ability to control autoreactive T cells that have escaped thymic selection.
Our laboratory studies the mechanisms that control T-cell tolerance in order to develop strategies to prevent and treat autoimmune diseases.
Overview
The thymus ensures a continuous production of naïve T lymphocytes, which are critically involved in the host defense against pathogens and malignant cells. Thymic epithelial cells (TECs) constitute an essential component of the stroma by providing indispensable clues for the differentiation and selection of thymocytes. In particular, the thymic medulla, composed of a dense network of medullary thymic epithelial cells (mTECs) and dendritic cells (DCs), provides a specialized microenvironment dedicated to the induction of T-cell tolerance. This microenvironment sustains the deletion of thymocytes bearing highly self-reactive T-cell receptors (TCRs), a process called negative selection (also known as clonal deletion). Although remarkably efficient, this process cannot completely purge the TCR repertoire of self-reactive specificities. The thymus thus produces a distinct T-cell subset called regulatory T cells (Tregs) that control potential deleterious effects of autoreactive T cells that have escaped the negative selection.
Our team studies the mechanisms that sustain the differentiation of mTECs and the generation of Foxp3+ Tregs. We also investigate the mechanisms of thymic regeneration with the objective to develop innovative strategies to boost T-cell immunity in many physio-pathological conditions in which the thymus has been damaged. Our research program has the following axes:
Impact of thymocytes on mTEC biology in physio-pathological conditions
Medullary thymic epithelial cells (mTECs) play a privileged role in the induction of T-cell tolerance by their unique ability to express thousands of self-antigens that are regulated in part by two transcription factors, Aire (Autoimmune regulator) and Fezf2 (Fez family zinc finger 2). The presentation of these self-antigens by mTECs and DCs leads to the deletion of potentially hazardous T cells and the generation of Foxp3+ Tregs. Recent advances have revealed that mTECs constitute a highly heterogeneous cell population with distinct functional properties in both mouse and human. We are studying the impact of developing T cells on mTEC heterogeneity in distinct physio-pathological conditions.
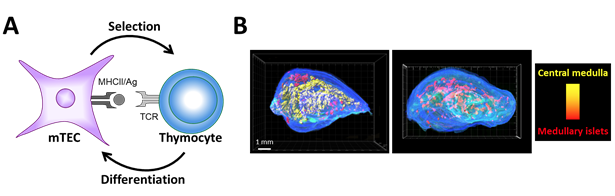
Concept of thymic crosstalk. (A) While mTECs control thymocyte selection, reciprocally thymocytes regulate mTEC differentiation. (B) 3D reconstruction of thymic lobes from wild-type mice (left panel) and H2A-a-/- mice (right panel) lacking CD4+ thymocytes using our homemade algorithm For3D (Full organ reconstruction in 3D). Note that the large central medulla is lost in absence of CD4+ thymocytes. Medullary islets are color coded according to their volume, from red (smallest) to yellow (largest). Copyright: Irla et al. Journal of Immunology 2013.
Development and suppressive activity of Tregs
The thymic medulla sustains the development of Foxp3+ regulatory T cells (Tregs). These cells, endowed with immunosuppressive activities, critically maintain immune homeostasis and prevent autoimmunity. This medullary microenvironment provides instructive signals (self-antigens, co-stimulatory molecules, cytokines) for their differentiation. Thymic Tregs are heterogeneous with two distinct precursors that give rise to mature Foxp3+ Tregs capable of controlling different types of autoimmune reactions according to the precursor from which they are derived. We are studying the molecular and cellular mechanisms that control Treg development.
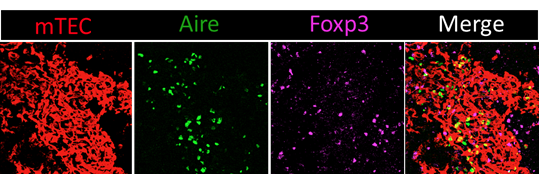
Confocal microscopy image of a thymic section stained with antibodies against the mTEC-specific marker K14 (red), Aire (green) and Foxp3 (magenta). Tregs are mainly localized in the medulla that contains Aire+ cells. Copyright: Magali Irla, CIML.
Deficiencies in Treg development or function result in uncontrolled immune responses that can lead to tissue destruction and inflammation. Remarkably, since Tregs use several modes of action to suppress immune responses, they constitute attractive therapeutic candidates in cell therapy. Indeed, recent advances have proven the feasibility, tolerability and efficacy of Treg-based therapy to treat autoimmune and inflammatory disorders and to induce tolerance in transplantation. We aim at identifying molecules that control Treg suppressive activity in order to facilitate their therapeutic use in the clinic. We use several preclinical mouse models of autoimmunity and transplantation.
Regeneration of the thymic function
The thymus is highly sensitive to several physio-pathological conditions such as the natural process of aging, myeloablative conditioning used to treat hematological disorders by bone marrow transplantation, graft versus host disease (GvHD) or infections. These different conditions induce severe damages on TECs and alter the recruitment of circulating T-cell progenitors, which impairs the thymic-dependent production of naïve T cells. Importantly, these damages could result in severe clinical complications characterized by an increased susceptibility to opportunistic infections, autoimmunity, tumor relapse, or the development of secondary malignancies. Nevertheless, the thymus is prone to regenerative therapies.
We are studying the molecular mechanisms responsible of thymic regeneration in order to develop innovative strategies to boost or recover T-cell production. Our studies are expected to improve immunity in many patients in which the thymus has been damaged.