TLRs are germline-encoded pattern recognition receptors that sense conserved molecular structures produced by microorganisms and play an essential role in host defence to microbial infection.
Interestingly, certain TLRs can recognise self-ligands as well and induce autoimmunity. Upon stimulation, TLRs activate intracellular signalling pathways that rapidly induce the expression of a variety of overlapping and unique genes involved in immune responses and inflammation.
New insights into TLR stimulation and function open new avenues for the treatment of infectious diseases, inflammation and autoimmunity.
Overview
A remarkable feature of the innate immune system is its ability to recognize invading microorganisms and to trigger a host defense response. Of particular interest are the recently identified Toll-like receptors (TLRs), which show homology with the Drosophila Toll protein and the interleukin-1 receptor family.
The TLRs are transmembrane proteins characterized by the presence of extracellular leucine-rich repeats and an intracellular Toll/interleukin-1 receptor-like domain. The mammalian TLR family consists of 13 members (TLR1-TLR10 in humans, TLR1-TLR9 and TLR11-TLR13 in mice) and each member recognizes evolutionary-conserved molecular structures unique to microorganisms. This specificity allows the TLR proteins to detect the presence of infection and to mediate overlapping, yet distinct, intracellular signaling pathways that lead to the induction of inflammatory and innate antimicrobial immune responses.
TLRs are expressed on various immune cells, including dendritic cells, macrophages, B and T cells and even non immune cells such as epithelial cells and fibrobalsts, and are localized on the cell surface or intracellularly. The TLRs that are expressed on the cell surface (TLR1, 2, 4, 5 and 6) largely recognize microbial membrane components, whereas the TLRs that are found almost exclusively in intracellular compartments (TLR3, 7, 8 and 9) recognize nucleic acids.
There are increasing evidence that intracellular TLRs can also recognise self-antigens released from damaged or stressed host tissue and that such recognition can lead to the development of autoimmune diseases, including systemic lupus erythematosus (SLE). Thus, in mammals TLRs are major triggers of inflammatory responses to microbial components or “danger” signals released from injured host cells that orchestrate both the innate and adaptive responses to eliminate infectious agents and control inflammation.
In our group we are exploring the role of mammalian TLRs in immunity using as animal models transgenic mice, such as TLR-deficient mice that we have generated by gene-targeting. Some of our recent findings are as follows:
Implication of endosomal TLRs in autoimmunity
Although TLR7 and TLR8 are phylogenetically and structurally related, their natural ligand viral single-stranded RNA stimulates human TLR7 and TLR8 and mouse TLR7, but not mouse TLR8, leading to the belief that TLR8 is biological inactive in mice. However, our recent work demonstrated an important and previously unidentified role for TLR8 in the regulation of TLR7-mediated autoimmunity in mice. Loss of TLR8 is accompanied by higher TLR7 expression and responsiveness in murine dendritic cells (but not in macrophages).
TLR8 deficiency in mice results in splenomegaly, reduced numbers of marginal zone and B1 B cells, systemic autoimmunity and glomerular deposition of IgM and IgG. Interestingly, these phenotypes can be rescued in double TLR8/TLR7 deficient mice (Demaria et al., J Clin Invest, 2010, 120: 3651). Our results suggest that in mice TLR8 expression negatively regulates TLR7 activity, and this is an important function of TLR8 since uncontrolled TLR7 expression can lead to autoimmunity (see figure below).
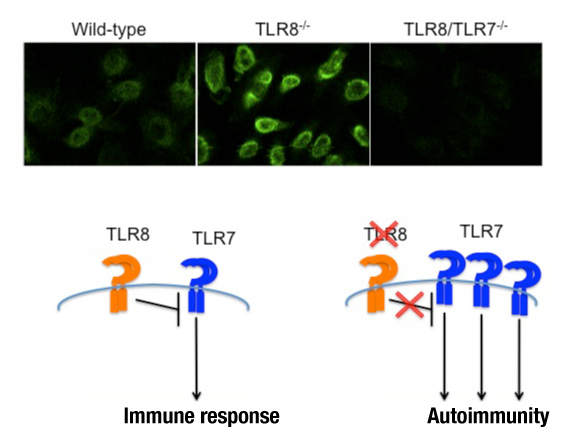 Upper panel: ANA staining patterns on Hep2 cells for wild-type, TLR8-/- and TLR8/TLR7-/- mouse sera. Lower panel: TLR8 deficiency in mice leads to lupus autoimmunity due to uncontrolled TLR7 expression and signaling.
Upper panel: ANA staining patterns on Hep2 cells for wild-type, TLR8-/- and TLR8/TLR7-/- mouse sera. Lower panel: TLR8 deficiency in mice leads to lupus autoimmunity due to uncontrolled TLR7 expression and signaling.
Moreover, in a different set of experiments we have addressed the implication of TLR3, TLR7 and TLR9 in autoimmune myocarditis. We showed that the resistance of MyD88-deficient mice to experimental autoimmune myocarditis (induction of cardiac inflammation upon immunization with cardiac α-myosin and complete Freund’s adjuvant) could be attributed to a certain degree to TLR7 signaling.
However, in the model of MCMV-induced myocarditis the severity of myocardial inflammation was higher in TLR9- and MyD88-deficient mice compared to TLR7-deficient and wild-type mice and paralleled the ability of the mice to fight the viral infection. So both TLR7 and TLR9 are implicated in autoimmune myocarditis, but their degree of contribution can vary depending on the experimental model that is studied (Pagni et al Autoimmunity 2010).
Contribution of TLR5 in the recognition of flagellated bacteria
Among TLRs, TLR5 is the receptor for flagellin, the major constituent of bacterial flagella and a virulence factor for Gram-negative and Gram-positive bacteria. We showed that in contrast to wild type, TLR5-deficient cells or mice where totally unresponsive to flagellin stimulation suggesting that TLR5 is essential for the recognition of bacterial flagellin both in vivo and ex vivo and that TLR5-deficient mice provide a unique model for studying the role of TLR5 in host defence against flagellated bacteria.
To elucidate the role of TLR5 in antibacterial host defence, we used two different flagellated bacteria Salmonella typhymurium and Pseudomonas aeruginosa, both common human pathogens.
We found that TLR5 contribution to antibacterial host defence to intraperitoneal infection with S. typhimurium or intranasal administration of P. aeruginosa may be masked by the presence of a functional TLR4 gene, since although TLR5-deficient mice behaved similar to wild-type controls, double TLR4/TLR5-deficient mice were more susceptible than single TLR4- or TLR5-deficient mice. T
Thus, cooperation of the TLR5 and TLR4 signalling pathways is vital for host defense against flagellated Gram-negative bacteria (Feuilet et al., PNAS 2006, 103:12487).
Future research in our laboratory focuses on the way in which the TLRs direct the immune responses to infectious agents, inflammation and self-tissues (autoimmunity). New insights into the mechanisms by which TLRs direct immune responses are changing the way we think about pathogenesis and open new avenues for the development of novel methods for the treatment of inflammation, infectious diseases and autoimmunity.