The fundamental goal of our research program is to contribute to cancer prevention monitoring and therapy, through the study of pathological deregulations of lymphocyte differentiation. We particularly seek to:
- Determine the basis of V(D)J-mediated genomic instability and cancer
- Decipher the stepwise malignant progression leading to lymphoma/leukemia development
- Identify risk factors and associated biomarkers
- Characterize oncogenic networks and novel therapeutic targets
- Define subgroups of patients which could benefit from targeted therapy
Overview
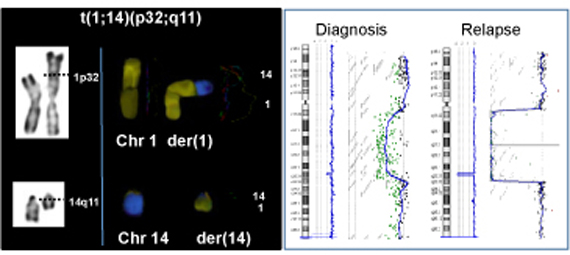
Lymphoid neoplasia is tightly associated with a wide range of chromosomal aberrations, a large number of which has been shown to be due to mistakes generated in the course of the V(D)J- or AID-mediated recombination. Such aberrations mostly consist of chromosomal translocations, inversions and micro-deletions occurring at the vicinity of proto-oncogenes/tumor suppressors and leading to their deregulated expression.
We and others have demonstrated the presence of 2 distinct mechanisms of V(D)J-mediated translocation in lymphoid neoplasia (Jaeger 2000; Welzel 2001; Nadel 2001; Marculescu 2002a, 2002b, 2003, 2006, Vanura 2009): one specifically involved in T-cell acute lymphoblastic leukemia (T-ALL), and the second in both T-ALL and few B-cell Non-Hodgkin lymphoma subtypes. This data constitutes a first step in defining the regulatory forces acting upon the genomic instability leading to B and T-cell tumorigenesis. During the last few years, we have taken advantage of our fine understanding of the mechanisms of chromosome aberrations in these human hemopathies to engage one step further towards the complex issue of oncogenic activation and stepwise neoplastic development, throughout 3 lines of research:
1- Follicular Lymphoma (FL), pathogenesis & associated risk factors
In a study interfacing epidemiology and molecular biology, we have demonstrated that clonotypic t(14;18) translocations - the hallmark of follicular lymphoma (FL) - can persist over a 10 year period in the peripheral blood from healthy individuals (Roulland 2006a, Lecluse 2009). Contrary to the current dogma, we further demonstrated that such translocations are actually carried by an expanding population of atypical follicular B-cells prone to constitute potent pre-malignant FL niches (Roulland 2006b, 2008, Agopian, 2009).
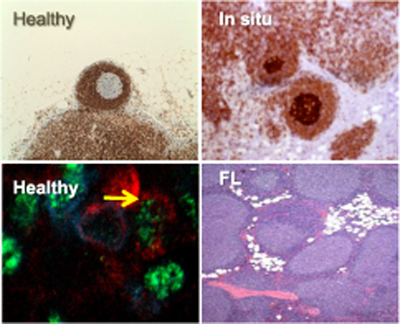
These findings strongly impact on the current understanding of disease progression, and on the proper handling of t(14;18) frequency in blood as a potential early biomarker for lymphoma.
2- Pathogenesis of T-cell Acute Lymphoblastic Leukemia (T-ALL)
To date, the subtle fine-tuning of a number of key oncogenic events within the complex multilayered networks operating in T-ALL has set many obstacles to the understanding of drug efficacy and resistance. In collaboration with multi-centric clinical institutions, we embarked into the fine and systemic characterization of biopsies from T-cell acute lymphoblastic leukemia (T-ALL) patients. Our findings of a large functional oncogenic network centered on the powerful oncogene c-MYC, raises the important question of the accurate definition of leukemia subtypes, and of the use of such criteria in the development of tailored therapy (Bonnet, 2011).
Through our integrative systemic, in depth, and functional approaches, our program should offer new perspectives on the complex connection between c-MYC and the other major oncogenic hubs in T-ALL, and contribute to the rational design of personalized therapy.
3- V(D)J-Mediated genomic instability
With the growing effort of refined subtyping, new oncogenes have recently emerged in the T-ALL landscape (Clappier 2007), and it has become increasingly clear that multiple oncogene cooperation occur in a large diversity of pathways (Vanura 2009, Dik 2007, Fischer 2007). Nevertheless, a large fraction of T-ALL still display mono-allelic oncogenic activation in absence of identified cytogenetic abnormalities, suggesting the presence of additional mechanisms of chromosomal alteration. In the hunt for such mechanisms, episomal circles have recently drawn particular attention (Vanura 2003, Marculescu 2003).
Although it has long been thought that signal-joints (SJ), the by-products of V(D)J recombination, are not involved in the dynamics of the rearrangement process, we demonstrate that SJ-mediated re-integration of excised episomal circles by the V(D)J recombinase might constitute an additional potent source of cancer in leukemia/lymphoma (Montpellier 2007).