Exploring the functions of the immune sentinels at the brain borders to control neuroinflammation
The Central Nervous System (CNS) is protected by the meninges, a three-layer covering that provides a structure onto which a myriad of resident innate immune sentinel cells block threatening pathogens or activate the adaptive immune system in response to inflammatory challenges. The recent discovery of this complex and dynamic meningeal innate immunity means that the development of new targeted therapeutic agents for the treatment of neurological disorders is within the realm of possibility.
“Rejane Rua and her team aim to unravel the remarkable complexity and plasticity of the brain immune sentinels’ functions in health and disease.”
The Central Nervous System (CNS) consists of the brain and the spinal cord and is protected by the meninges, a protective three-layer covering formed of the dura mater, the arachnoid mater and the pia mater. The space between the arachnoid and pia maters is filled with the cerebrospinal fluid that protects, nourishes and cleanses the CNS. Once thought to merely shield the CNS parenchyma containing mostly neurons and glial cells, the meninges have been proven to harbor a large network of functions, owing to the presence of newly identified cells including cells of the immune system, particularly novel innate immune sentinel cells.
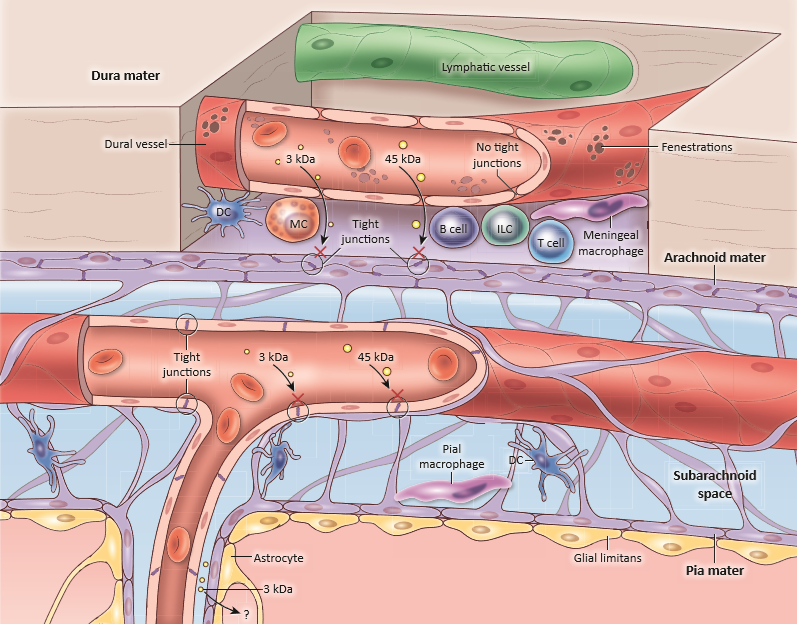
Schematic diagram of the steady-state meningeal anatomy showing the three layers (dura, arachnoid and pia) covering the brain surface. Immune composition showing the large repertoire of immune sentinels such as the Dendritic Cells (DCs), Mast Cells (MCs), Innate Lymphoid Cells (ILCs) and Meningeal Macrophages (MMs).
Copyright: Rejane Rua and Dorian McGavern, Trends in Molecular Medicine.
Balancing the inflammatory reaction is key to maintaining the physiology of a healthy CNS but achieving this balance is not such a straightforward process; a small divergence can disrupt the CNS homeostasis and lead to harmful effects. A reaction that breaks the immune tolerance to self leads to autoimmune disorders such as Multiple Sclerosis (MS), an inflammatory demyelination disease. On the other hand, immunosuppression can lead to pathogen neuroinvasion and CNS infections. Since the immune sentinel cells play a central role in regulating the neuroinflammation, a detailed anatomical and functional exploration of the meninges is paramount to understanding their precise roles. Rejane Rua and her team are using cutting-edge technologies such as intravital imaging, histo-cytometry, functional flow cytometry and single-cell transcriptomic approaches that together allow for a precise and dynamic investigation of the meninges in wildtype and transgenic mice models. They aim to unravel the remarkable complexity and plasticity of the immune sentinels’ functions in health and disease.
The CD4+ T helper 17 (TH17) cells and the T-box transcription factor T-bet play a critical role in driving MS and Experimental Autoimmune Encephalomyelitis (EAE), the mouse model of the disease. However, the precise mechanisms by which these cells leave the meninges and perivascular spaces to infiltrate the parenchyma and start a cascade of events leading to the demyelination of the neurons were until recently unknown. Using transgenic mice models lacking immune sentinels, histo-cytometry and confocal microscopy, Rejane Rua and her colleagues showed that a specific type of immune sentinels, the T-bet-dependent NKp46+ Innate Lymphoid Cells (ILCs), controlled the CNS parenchymal infiltration. Those sentinels indeed created a deleterious pro-inflammatory cytokine environment in the meninges. These findings clearly demonstrate that the meningeal immune sentinels have a key role in initiating the neuroinflammation and thus have a detrimental role in the CNS integrity. This work was profiled in the cover of the prestigious journal Nature Immunology.
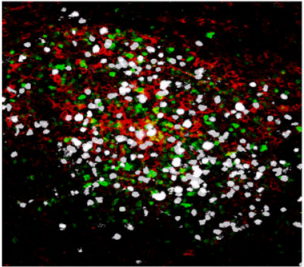

Confocal microscopy images of meningeal whole mount showing an inflammatory cluster, containing auto-reactive CD4+ T cells TH17 (white), antigen-presenting cells (red) and innate lymphocytes (green) at the peak of the EAE disease.
Copyright: Rejane Rua and Vanja Lazarevic, Nature Immunology. Artwork illustrating inflammatory clusters. Copyright: Lewis Long, Springer Nature Publishing AG.
In addition to lymphoid sentinels, the surface of the CNS is also inhabited by a vast network of myeloid sentinels. Resident Meningeal Macrophages (MMs) are the most abundant immune cells in the meninges and are constantly sampling their environment. However, little is known about how they are maintained and regulated during and after an inflammatory challenge. By following these cells upon infection by the lymphocytic choriomeningitis virus (LCMV) that causes meningitis, Rejane Rua and her colleagues showed that the pool of these specialized MMs is greatly altered, thus leading to long-term defects in their immune function. Using genomic, intravital imaging and cell tracking approaches, they established that during viral meningitis, the MMs are killed by infiltrating cytotoxic T lymphocytes (CTLs), cleared and replaced by peripheral monocytes that differentiate upon exposure to the cytokine Interferon-Gamma. Engrafted cells are impaired as shown by a loss of bacterial and immunoregulatory sensors that could lead to recurrent infections or susceptibility after big episodes of inflammation.
Representative time lapses of 3D reconstructions show the dynamics of myeloid cells (green) captured by intravital imaging through a thinned skull of naive Cx3cr1-GFP/+ mice
(Top panel with video link showing steady state MMs) and through a thinned skull of LCMV-infected Cx3cr1gfp/+ mice at the peak of the disease where the LCMV-specific CTL express a fluorescent protein (pink)
(Bottom panel with video link showing interactions between MMs and CTLs at the peak of the disease). The blood vessels are labelled with Evans blue (red). Copyright: Rejane Rua et al., Nature Immunology.
Regulating the kinetics and amplitude of the meningeal immune responses may be used in the future for the treatment of neuroinflammatory disorders.
Both the activation of ILCs in the development of MS and the engraftment of monocytes during meningitis impair CNS integrity. In one case demyelination following meningeal and CNS inflammation has debilitating effect on the CNS function, in the other case the impairment of MMs induces a susceptibility to subsequent infections. These findings open new opportunities for the development of novel therapies by targeting these meningeal immune sentinels. Reducing the potency of the ILCs would avoid causing deleterious neuroinflammation seen in MS. Restoring the pool of some resident MMs would impede the loss of immune function after an inflammatory challenge. Despite clinical and experimental evidence showing the role of the meninges in the control of neuroinflammation, the pathways by which immune cells control CNS inflammation are understudied; Rejane Rua and her team have already started to shed some light onto the complex interplay of meningeal immune cells and neuroinflammatory events.