Decoding human germinal center B cells program in health and disease to design new anti-tumor therapies
Germinal centers (GCs) are microscopic power houses in which B cells proliferate and are prompted to produce high-affinity antibodies that neutralize invading pathogens, keeping us from developing life threatening infectious diseases. The remarkable anatomical, cellular and molecular plasticity of GCs are highly coordinated; any disruption may alter the response to pathogenic microbes and could favor the formation of cancers. Decoding the precise molecular dynamics by which GCs operate is key to developing new and targeted therapies for challenging infections and cancers.
“Numerous findings have shed some light on GC biology, but the integrative single-cell approach taken by the team of Pierre Milpied is transforming our understanding.”
Germinal centers (GCs) are transient little compartments arising in the follicles of secondary lymphoid organs such as the spleen and the lymph nodes. They appear after exposure to pathogenic microorganisms and house the immune reaction in which GC B cells undergo repeated cycles of proliferation, selection and differentiation to produce antibodies-secreting plasma cells and memory B cells, their ultimate achievement, as those long-lived cells will provide sustained protection. Once the immune response has cleared the pathogens, GCs are terminated, and the follicles are reestablished. Numerous findings have shed some light on GC biology, but the integrative single-cell approach taken by the team of Pierre Milpied is transforming our understanding.
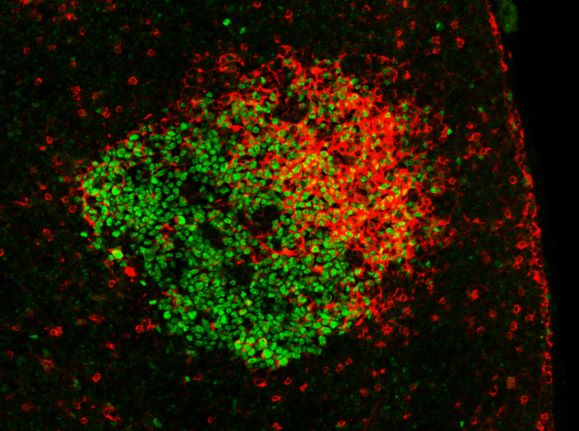
Germinal center in a mouse lymph node after immunization with a model antigen. GC B cells express the BCL6 transcription factor (green). Antigen-specific antibodies (expressed on B cells as BCR or captured on the follicular dendritic cell network) contain a lambda 1 light chain (red). Copyright: Pierre Milpied, CIML.
Much of the current knowledge on GC biology is based on bulk measurements and averages that reflect dominant biological mechanisms in a population of cells. However, this approach does not factor in behavioral differences between cells within the studied population. Knowing that cellular heterogeneity plays a critical role in defining GC biological processes, it is important to develop new and tailored strategies to assess GC functional and molecular plasticity with single-cell resolution. With the aim of integrating a comprehensive map of phenotypic and gene expression changes within a model of GC B cell dynamics and differentiation, Pierre Milpied and his team have chosen to study the GC reaction at the single-cell level. Using powerful bioinformatics tools, they have been integrating several high-throughput data sets from GC B cells (transcriptome, phenotype, antibody gene sequence) to decipher B cell molecular programs in healthy and cancerous tissues.
The integrative B cell immunology team of Pierre Milpied is engaged in modeling germinal centers in health and disease and in decoding human cancer-infiltrating B cell responses.
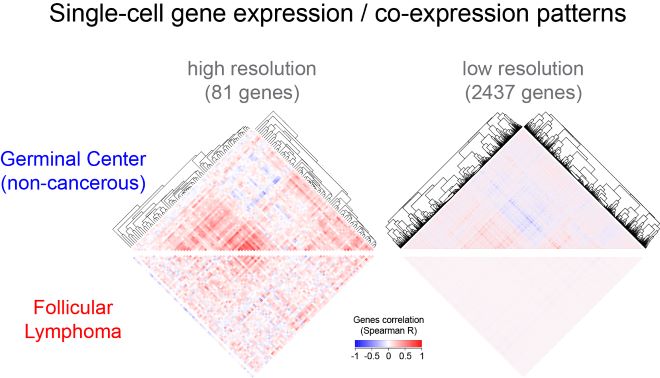
De-synchronization of GC gene expression programs in B cell lymphoma. Clustered gene correlation heatmaps of single-cell gene expression highlight characteristic patterns of genes co-expression in GC B cells (top). Those gene correlation patterns are lost in lymphoma B cells (bottom). Copyright: Pierre Milpied, CIML.
Although GC B cells transition through multiple states during the GC reaction, they are classically categorized into two subsets according to their location, DZ (Dark Zone) or LZ (Light Zone). Integrative single-cell analysis of gene expression, phenotype and antibody gene sequencing has revealed that human GC B cells exist as a continuum of transitional states rather than as segregated DZ and LZ populations. Remarkably, GC B cell transcriptional heterogeneity was characterized by synchronized gene clusters. Those findings were then used as a baseline to study one of the most common adult lymphomas, follicular lymphoma (FL), a GC B cell-derived cancer that displays transcriptomes closely related to those of normal GC B cells. Using the same integrative single-cell approach that provided unique GC B cell expression signature and gene clustering, Pierre Milpied and his colleagues discovered a “de-synchronization of GC gene expression programs” in the B cell lymphoma; their work was published in the prominent journal Nature Immunology in September 2018.
Not only does this integrative single-cell analysis provide insight on how to optimize diagnosis, prognosis and therapy for B cell lymphomas, it can also be applied to study solid cancers that have been shown to host cancer-infiltrating B cells in organized structures resembling GCs. The team of Pierre Milpied is developing a pipeline to define the antigen specificity and potency of antibodies derived from in situ B cell responses, in the hope of identifying new ways of stimulating pre-existing anti-tumor neutralizing responses.
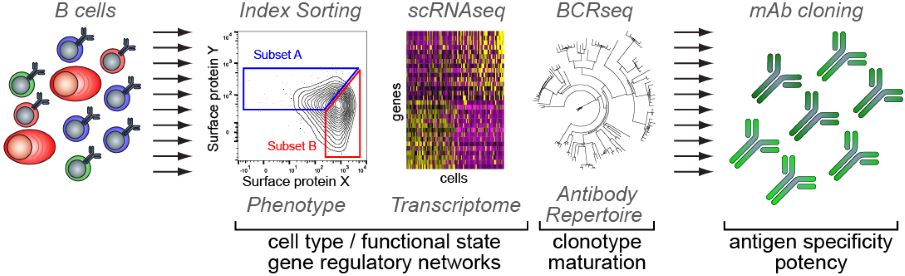
Pipeline for the integrative single-cell analysis of B cells Copyright: Pierre Milpied, CIML.
The work of Pierre Milpied and his team sets the stage for the development of personalized medicine.
More than simple power houses, germinal centers provide a platform and microenvironment for generating B cells with high functional heterogeneity and plasticity. Using innovative approaches, the team of Pierre Milpied is deciphering the molecular code of GC biology, giving us insight on how to design personalized and precision medicine to treat many lymphomas and solid cancers.