Deciphering the early events of B cell activation upon infection to generate novel vaccination strategies
B-cells, along with a myriad of other immune cells, participate in protecting individuals from getting seriously sick upon exposure to pathogens. B cells keep us from harm by secreting specific molecules known as antibodies, which can neutralize pathogenic microbes during infection. Deciphering how and when those protective antibodies are generated and the immune network regulating their production, is central to develop alternative strategies for vaccination.
“The dynamic cellular network of lymph nodes provides a scaffolding onto which B-cells hitch-hike to arrive at a central station, the germinal centers, where they are prompted to produce protective antibodies”
B cells initially encounter infectious pathogens in small organs called lymph nodes. These organs are found all over the body and they are the ones that swell when we become sick, a proof that our immune system is functioning well. We knew that after making contact with foreign microorganisms, B-cells need to travel to different zones of the lymph nodes and collect signals necessary to start producing antibodies. However, we didn’t exactly know the nature and source of the signals required by B-cells to successfully move from one station to the following one. That is what Mauro Gaya and collaborators sought to find out and greatly succeeded: his work was profiled in the cover of the prestigious journal Cell.

Artwork illustrating NKT cells delivering innate signals (yellow) to activated B-cells that in turn produce protective antibodies upon infection.
Copyright: Rémi Duval, Cell.
The advent of cutting-edge imaging technologies and the ever-growing field of transgenics has led Mauro Gaya to unravel some enigmas of the intricate cellular network of the lymph nodes and to identify key players leading B cells on their path to antibody production. Mauro Gaya identified a small but important population of lymphocytes called Natural Killer T (NKT) cells, that provide early and localized signals to B cells, essential for their arrival at the central station, the “germinal centers”, where the refinement of antibody fabrication takes place.
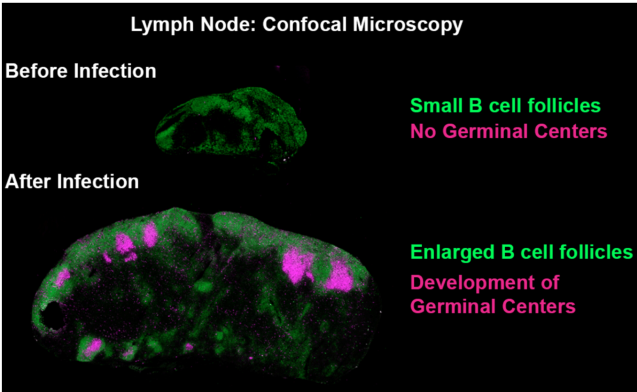
Confocal microscopy images showing the formation of germinal centers after exposure to a pathogen. Green: B220 Pacific Blue, Magenta: GL-7 Alexa Fluor 647. Copyright: Mauro GAYA, Cell.
“Boosting our immune responses during a primary infection can leave us temporarily susceptible to opportunistic secondary pathogens”
One of the first symptoms we experience when we have an ongoing infection in our body is our lymph nodes getting bigger. The increase in the size of these organs allows the accommodation of large numbers of immune cells ready to fight the invading microorganisms. This phenomenon is tightly regulated and involves a dramatic remodeling of the lymph node architecture. We found that as part of this remodeling, the layer of macrophages placed at the outer layer of lymph nodes is temporarily disrupted. As this macrophage layer is essential to capture and retain pathogens entering into the lymph nodes, the disruptive phenomenon observed during primary infection prevents the node from processing a second round of pathogenic exposure until these macrophages are back and have reestablished their protective state. If the lymph nodes momentarily cease to properly operate after a given pathogenic attack, it means that B cells won’t produce new neutralizing antibodies for some time, leaving the host not fully protected against secondary opportunistic microbes. What does this all mean for our protection against a subsequent pathogenic exposure? How can we use this information to generate better vaccination schedule? Even better vaccine strategies?
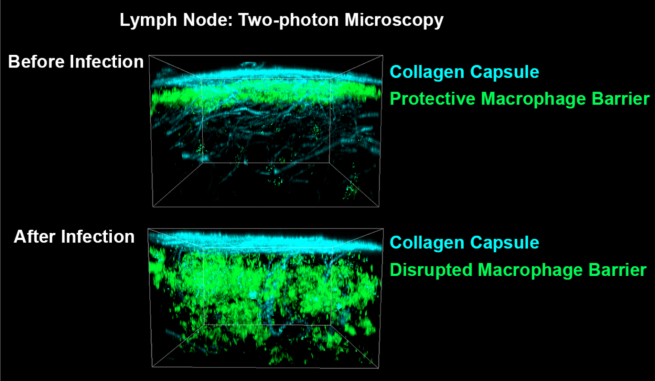
Two-photon microscopy 3D projections showing the migration of SCS macrophages (green) inward after exposure to inflammatory signals. Cyan: collagen, second harmonic signal. Green: CD169, Alexa Fluor 647. Copyright: Mauro GAYA, Science.
The work of Mauro Gaya gives us some insight on how to optimize vaccination.
Vaccination is a process by which individuals are rendered immune to developing serious diseases by the administration of vaccines. The widespread immunity that vaccination conveys has led to worldwide eradication of smallpox and the elimination of diseases such as polio and diphtheria from most parts of the world. In active vaccination, the vaccine contains modified antigens and individuals activate their own immune system to synthetize their own antibodies. In passive vaccination, the vaccine contains preformed antibodies and individuals do not produce their own stock, relying solely on infused antibodies.
At present, the active vaccination is the most desirable. Therefore, most currently licensed human vaccines rely on the induction of high affinity antibodies that can neutralize infectious pathogens in case of future exposures. However, the generation of vaccines against certain types of viruses, such as influenza and HIV, fail to provide long-lasting protection due to viruses’ rapid accumulation of mutations. Owing to the lack of cross-protective vaccines against the different strain variants, there is an immediate medical need for new therapeutic approaches that can effectively protect us from this kind of viruses. A deeper understanding of the cellular and molecular mechanisms of B cell activation in response to infection is thus key in the development of next-generation vaccines.
"Can the generation of broadly protective vaccines be derived from a functional study on how B cell immunity is triggered during infection?" By taking advantage of cutting-edge imaging techniques, multi-parametric flow cytometry, in vivo infection models and the generation of new transgenic mouse lines, the team of Mauro Gaya will provide insightful data on how to revise vaccination strategies to best tailor the needs of clinicians.