How and where do B cells grow?
B cells are a type of lymphocyte specialized in the production of antibodies, proteins designed to recognize elements (or antigens) carried by foreign agents in the body, permitting them to be recognized by the immune system.
B cells are continuously generated in the bone marrow in the form of inexperienced precursors. Step by step, they acquire different properties, the first being not to react against normal components of the individual (the self). The following steps change them into mature cells, each capable of producing antibodies recognizing a particular antigen.
The first stages of this development, called differentiation, occur in the bone marrow, the later stages happen elsewhere, particularly in the spleen, the lymph nodes and the mucosa-associated lymphoid tissues.
Specializing in early life stages of B cells, Claudine Schiff's team has developed tools and systems for studying early stages of B cell differentiation in bone marrow.
Within the marrow, pre-B cells are contained in a meshwork consisting of stromal cells (mixed connective tissue cells, fat cells and young bone cells). Claudine Schiff and her team have shown that these pre-B cells could not start their differentiation without the aid of these stromal cells.
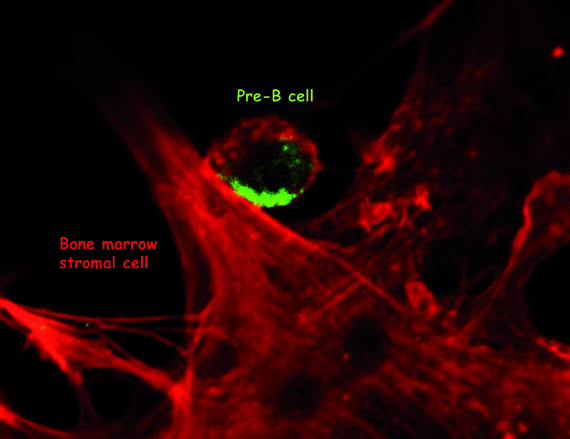
The pre-B cells (in green) are localized in specialized “niches” in the marrow composed of stromal cells (in red). Copyright Claudine Schiff, CIML.
"The pre-B cells are localized in specialized “niches” in the marrow composed of stromal cells with which they establish very close contacts," says Claudine Schiff. "So the stroma not only structure the bone marrow, it also play an active role in secreting molecules such as galectin 1, which induce pre-B cells to differentiate. Pre-B cells possess only one incomplete antigen receptor, but this is already sufficient to receive information that will guide their maturation."
Towards the first functional map of the bone marrow?
It’s impossible to reconstruct the stromal niches and their dynamics in a test tube; they lose all their properties in vitro. However, if we reinject into mice pre-B cells selected at a given stage of differentiation, they will spontaneously become localized in these famous niches in which we visualize unambiguously the protein galectin 1, produced by stromal cells, and the pre-B cell receptor for antigen.
"Neither younger B cell precursors nor more differentiated B cells have the same tropism for these niches, in which one specific stage of maturation takes place," says Claudine Schiff. "Soon, we will be able to identify other types of stromal niches, formed to support other phases of the differentiation of B cells.” They hope to not only design a functional map of the bone marrow but also reveal the factors involved during the medullary differentiation of B lymphocytes.
These findings are supported by observations made in patients with agammaglobulinemia, an immune deficiency of genetic origin, which results in an absence of circulating antibodies. Claudine Schiff's team has access to patient samples, in which the study of defective genes permits the confirmation of the role of known factors in the differentiation of B cells, but also the identification of novel factors, whose function will be then studied in the models developed by the team.
When the stroma also plays a pathological role
These studies on interactions between stromal and pre-B cells in the bone marrow also illuminate aspects related to pathological function of these stromal niches.
Lymph nodes, where much more mature B cells reside, have structures similar to those observed in bone marrow, structures that promote other lympho-stromal interactions. These are maintained - and play a deleterious role - at the onset of follicular lymphoma, malignant transformation of B cells residing in a node.
"In the majority of patients with follicular lymphoma, we found that cancerous B cells are present in the bone marrow very rapidly" Claudine Schiff observes. "It is not known yet whether these cells return from the lymph nodes to the bone marrow, but we find that they have reconstituted a stromal environment conducive to their growth. We study the mechanisms of migration "return" of these cancer cells to the marrow and the role of the stroma in the selection of tumor clones in these reconstituted niches. This is an issue that has major therapeutic implications, since nothing says that the lymphoma cells localized in the bone marrow are as sensitive to treatment than those who remained in the lymph nodes: all indications, however, indicate rather than the bone marrow plays a protective role vis-à-vis of these cancer cells."
Today, the team of Claudine Schiff may legitimately claim a unique understanding of cellular interactions that are involved in the earliest moments of the birth and differentiation of B lymphocytes. Now validated by clinical observations, these findings should contribute to a better understanding of the mechanisms facilitating the establishment and growth of cancer cells in the bone marrow.