Innate Immunity at mucosal sites
The mucosae represent the border between our body and the environment, and they act as the first barrier against infections. Therefore, inflammatory responses must be tightly regulated to combat infection without causing excessive self-damage or interfering with the repair process. An imbalance in these processes could result in the loss of barrier function and tissue functionality.
Achille Broggi and his team study the interplay between the immune system, the microbiota, and the mucosal layer.
In the team we are interested in :
a) understanding how IFN-λ production and functions are regulated in the intestinal mucosa and how they regulate the pathogenesis of IBD "
b) Exploring how malnutrition affects the mucosal immune response and how commensal microbe can influence the response to malnutrition.
c) Assessing how the metabolism of the CoA metabolite pantetheine by the enzyme Vnn1 affects immune and epithelial responses during mucosal inflammation.
Axis1: Exploring the role of type III interferons in the intestine.
P.I. Dr. Achille Broggi, Team Leader, CRCN-CNRS
The team is interested in the biology of type III interferons (or IFN-λ) , which is a class of antiviral cytokines that has risen to importance as a pivotal regulator of the mucosal responses.
IFN-λ induces a similar transcriptional signature to type I interferons, they are similarly produced in response to viral PAMPs and can stimulate transcription of antiviral genes in target cells.
However, since IFN-λ’s specific receptor (IFNLR1) is expressed mainly in cells of epithelial lineage and a selected pool of immune cells, IFN-λ is considered a specialized mucosal cytokine. Indeed, IFN-λ is known to act directly on epithelial cells to exert local antiviral activity, but we also made the surprising discovery that IFN-λ can downmodulate the inflammatory response to protect the mucosae. We showed that IFN-λ can inhibit neutrophil ability to initiate tissue-damaging responses, such as ROS production, and degranulation via non-canonical transcription independent mechanisms.
We also discovered that this ability of IFN-λ to downregulate neutrophil responses is evident during acute inflammatory conditions at mucosal sites such as during experimental DSS colitis. Notably we were among the first to describe a role for IFN-λ in the gut in the absence of an acute viral infection, where basal levels of IFN-λ are induced in response to commensal enteric viruses.
Given its pivotal role in the control of mucosal inflammation and epithelial cell responses, we are interested in the study of non-redundant and non-canonical signaling pathways of IFN-λ in the context of autoimmune inflammatory responses of the mucosae.
In particular, we are interested in the characterization of the role of interferons in barrier healing at mucosal surfaces, both in the context of acute viral infections as well as in the context of basal IFN levels stimulated by commensal microbes in the gut.
While the during acute respiratory infections, the ability of IFN-λ to limit immunopathology but maintain antiviral activity is believed to be beneficial to the maintenance of barrier function, we recently demonstrated that in the context of prolonged antiviral immunity activation, IFN-λ induces barrier damage, causing susceptibility to lethal bacterial superinfections.
In particular, we demonstrated that IFN-λ directly inhibits lung epithelial cell capacity to proliferate and regenerate barrier function. This defect in barrier function culminates in failure to tolerate opportunistic pathogen invasion and causes lethality upon bacterial superinfection.
These observations raise the question of whether IFN-λ produced in response to the gut microbiota in the gut may influence tissue recovery in the context of intestinal inflammatory diseases.
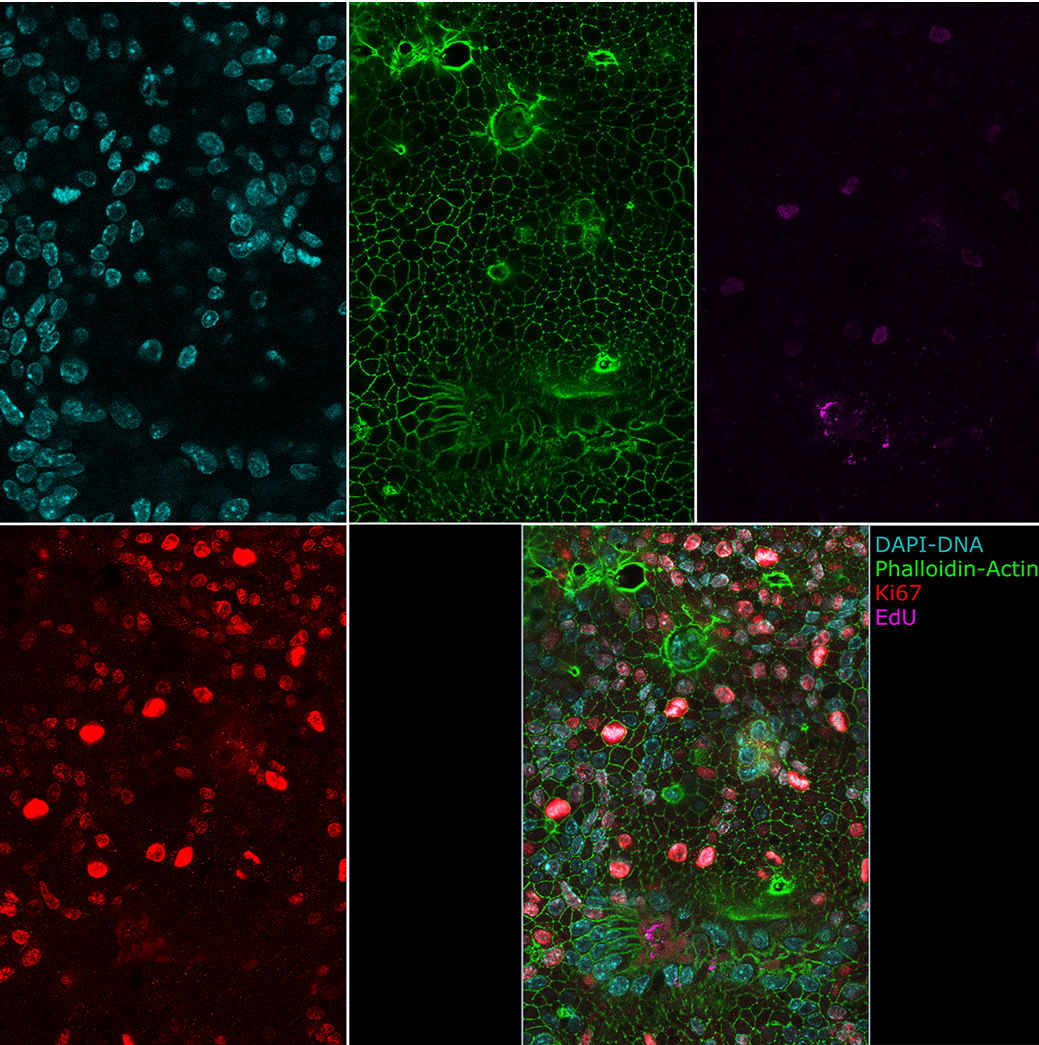
Intestinal Organoids grown in 2D at the air liquid interface. Cyan: DAPI, Green: Phalloidin, Magenta: EdU, Red: Ki67.
We will explore this question by using several different models, ranging from in vivo mouse models of IBD to in vitro models of epithelial biology such as human and mouse intestinal organoids and model of mouse and human immune cells.
Understanding how IFN-λ differently regulates the immune and epithelial response will be extremely useful to devise therapies able to intervene on the one hand to favor the anti-inflammatory role of IFN-λ, and on the other hand to interfere with its detrimental role during tissue recovery
Axis2: Understanding the effect of malnutrition on the immune response.
P.I. Dr. Julie Tomas, CRCN-CNRS
Early emergence of malnutrition has disastrous consequences later in life and must be assessed at very early stages to fully understand the pathogenesis and associated side effects such as recurrent infections and noncommunicable diseases. We developed a Severe Acute Malnutrition (SAM) mouse model in males starting at weaning that reproduces the main physiological, immunological, microbial and metabolic features of SAM in children. In addition, we have identified key markers to monitor under nutritional intervention: the mucosal immune system and the intestinal microbiota, which remain dysbiotic unlike physiological parameters.
Today, using this system, we are studying how to compensate for the side effects of SAM with probiotics, bacterial metabolites, combined or not with nutritional intervention, and how malnutrition favors infections. We are also investigating the differences in the response of malnourished mice to vaccination (Salmonella enterica Typhimurium) and infections (Enterobacteriaceae) to unravel the mechanisms involved and identify associated biomarkers.
Axis3: Assessing how the metabolism of the CoA metabolite pantetheine by the enzyme Vnn1 affects immune and epithelial responses during mucosal inflammation.
P.I. Prof. Franck GALLAND, PU-AMU
Together with Professor Philippe Naquet at CIML, Franck Galland initially identified the Vanin-1 pantetheinase (Vnn1) pathway as a key regulator of tissue resilience to stress in various mouse models and in the context of various human pathologies. Recent findings highlight the central role of Vnn1 as a colonocyte cytoprotectant, promoting mucus production and strengthening the intestinal mucosal barrier. Vnn1 has a dual impact on colon homeostasis: firstly, the by-products of its enzymatic activity, cysteamine and pantothenic acid (vitamin B5), play an essential role in promoting coenzyme A regeneration, facilitating metabolic reorientation and contributing to overall colonic health ; secondly, these by-products influence host-microbiota interactions, by specifically stimulating butyrate production by certain bacterial species. Butyrate is known to meet the energy requirements of the intestinal mucosa, and its depletion is a feature of inflammatory bowel disease (IBD). Consequently, the Vnn1-pantheinase pathway intricately shapes the metabolic well-being of the intestinal mucosa, safeguarding the integrity of colonic crypts and increasing resistance to colitis. Restoring the intestinal barrier is emerging as a new priority in the management of inflammatory bowel disease. Maintaining optimal levels of vitamin B5 throughout the course and treatment of IBD (e.g. anti-TNFα), which can be achieved by administering Vnn1 derivatives, could increase the efficacy of anti-inflammatory therapy and patient recovery. On the other hand, using the Vnn1 model, we are currently seeking to better understand how the metabolic state of the intestinal epithelium might impact gut immune regulation and, consequently, influence the response to enteric pathogens.