Innate Immunity at mucosal sites
The mucosae represent the border between our body and the environment, and they act as the first barrier against infections. Therefore, inflammatory responses must be tightly regulated to combat infection without causing excessive self-damage or interfering with the repair process. An imbalance in these processes could result in the loss of barrier function and tissue functionality.
Achille Broggi and his team study the interplay between the immune system, commensal microbes, and the mucosal layer. The team is interested in:
a) understanding how immune mediators production and functions are regulated in the intestinal mucosa and how they regulate the pathogenesis of inflammatory bowel disease (IBD) .
b) Exploring how malnutrition affects mucosal homeostasis and immune responses.
c) Determining how the metabolization of nutrients by the enzyme Vnn1 affect tissue repair and immune responses in the gut
Axis1: Exploring the role of type III interferons in the intestine.
P.I. Dr. Achille Broggi, CE CRCN
The mucosae represent our interface with the environment and the first line of defense against invading pathogens. Their function as physical barriers means that the inflammatory process at these sites must be tightly regulated to combat invading pathogens while limiting collateral damage to self-tissues. In a healthy individual, this balance allows for efficient repair and barrier reestablishment once the infection is resolved.
We found that a class of antiviral cytokines called Interferon Lambda (IFN-λ) has an important role in this context.
Indeed, while IFN-λ is mainly sensed by mucosal epithelia and can thus directly protect them from pathogenic viral infections, we also found that it has a specific regulatory activity on inflammatory cells (neutrophils).
A B
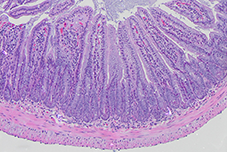
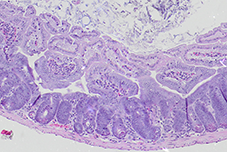
The intestinal crypt after radiation damage.
Histology slides depicting the mouse small intestinal mucosa of A) healthy mice, B) mice with epithelial damage following irradiation. After damage, intestinal epithelial cells proliferate and reconstitute tissue functionality. We are interested in the role of IFN-λ in this context.
This dual action of IFN-λ ensures the efficient control of viral pathogens and, at the same time, controls potential detrimental inflammatory reactions.
We have recently found, however that there is a dark side of IFN-λ. Indeed when IFN-λ is aberrantly expressed at the tail end of a viral infection, can impair the ability of epithelial cells to proliferate and repair damaged tissues., In these pathological conditions, IFN-λ can inhibit repair and the resolution of inflammation, which are needed to return to health.
This ability of IFN-λ to both protect the mucosae and, in pathological settings, to have the ability to be detrimental during repair, makes it an extremely interesting cytokine to study in the context of mucosal diseases.
In this context we are interested in studying the role of IFN-λ in intestinal inflammation, and how naturally occurring IFN-λ, produced in response to the intestinal flora, can impact the pathogenesis of autoimmune diseases of the gut such as IBD.
We will explore this question by using several different models, ranging from in vivo mouse models of IBD to in vitro models of epithelial biology such as human and mouse intestinal organoids and model of mouse and human immune cells.
Understanding how IFN-λ differently regulates the immune and epithelial response will be extremely useful to devise therapies able to intervene on the one hand to favor the anti-inflammatory role of IFN-λ, and on the other hand to interfere with its detrimental role during tissue recovery.
Axis2: Understanding the effect of malnutrition on the immune response.
P.I. Dr. Julie Tomas, CRCN
Combating malnutrition is one of the greatest global health challenges. Early emergence of malnutrition has disastrous consequences later in life and must be assessed at very early stages to fully understand the pathogenesis and associated side effects (recurrent infections and noncommunicable diseases). To overcome the limits of clinical research in malnourished children, we developed a mouse model of Severe Acute Malnutrition (SAM) that faithfully recapitulates key features of the malnourished states of humans. The availability of this mouse model allows the simultaneous assessment of physiological, metabolic, immune and microbiome dysregulations at mucosal sites. Moreover, we identified the mucosal immune response and the intestinal microbiota as key aspect to monitor to assess the effectiveness of nutritional intervention. To reduce the onset of malnutrition and its side effects, there is an urgent need to understand how current therapies can be improved and turned into preventive treatments. Taking advantage of this system, we found profound differences in the response of malnourished mice to vaccination and infection recapitulating observations in humans. In the team we study how to compensate for the side effects of malnutrition using probiotics, bacterial metabolites, combined or not with nutritional intervention. We are also interested in studying how malnutrition affects responses to vaccination and infections, to identify biomarkers and develop effective therapeutic strategies.
Axis3: Determining how the metabolization of nutrients by the enzyme Vnn1 affect tissue repair and immune responses in the gut.
P.I. Prof. Franck GALLAND, Prof.
Exposure of tissues to stress profoundly impacts their functionality, triggering the release of Danger-Associated Molecular Patterns (DAMPs) and causing a reallocation of energetic resources towards mechanisms that regulate tissue protection, repair, and regeneration. This restructuring of tissues disrupts the delivery of essential nutrients, oxygen, and cellular metabolism. Periods of tissue destruction alternate with phases of regeneration, and during these dynamic adaptations, changes in the concentration of intermediate metabolites are monitored by metabolic checkpoints. Certain metabolites are exchanged between cells, shaping microenvironments and influencing tissue resilience to stress, as well as immune adaptation. Franck Galland, in collaboration with Professor Philippe Naquet at CIML, has co-discovered a new family of "Vanin" molecules. The prototype of this family, Vanin-1 (Vnn1), functions as an epithelial pantetheinase, playing a crucial regulatory role in tissue stress tolerance across various experimental mouse models and human pathologies. Franck Galland is actively engaged in a research project that delves into the Vnn1/pantetheinase pathway, specifically in the context of cytoprotection and reconstruction of the intestinal mucosa in both mice and humans. This exploration sheds light on the significance of vitamin B5 metabolism in host-microbiota interactions and in preserving the integrity of the colonic intestinal barrier. Consequently, this research opens up promising therapeutic avenues for chronic inflammatory bowel diseases (IBD).