Here are the microscopes available on the platform for internal use and external collaborations, from single molecules to small animals
- Intravital and large sample imaging
- Molecular imaging
Cellular imaging
Spectral Confocal Laser Scanning microscopy
Confocal microscopy is a powerful technique that rejects out of focus light allowing optical sectionning.
The understanding complex immunological contexts requires the identifications of multiple cellular populations which is achieved in routine by using spectral confocal imaging allowing up to 10 color imaging.
Confocal microscopes offer options like highly sensitive spectral detectors and software options such as FCS (Fluorescence Correlation Spectroscopy), FRAP (Fluorescence Recovery After Photobleaching), RICS (Raster Image Correlation Spectroscopy) techniques.
Microscopes available :
LSM 880 with 32 GaAsP detector + 2PMTs + spectral detection + Fast AiryScan and lasers at 405, 458, 488, 561, 633nm.
LSM 780 with 32 GaAsP detector + 2PMTs + spectral detection and lasers at 405, 440, 458, 488, 561, 633nm.
Example of results :
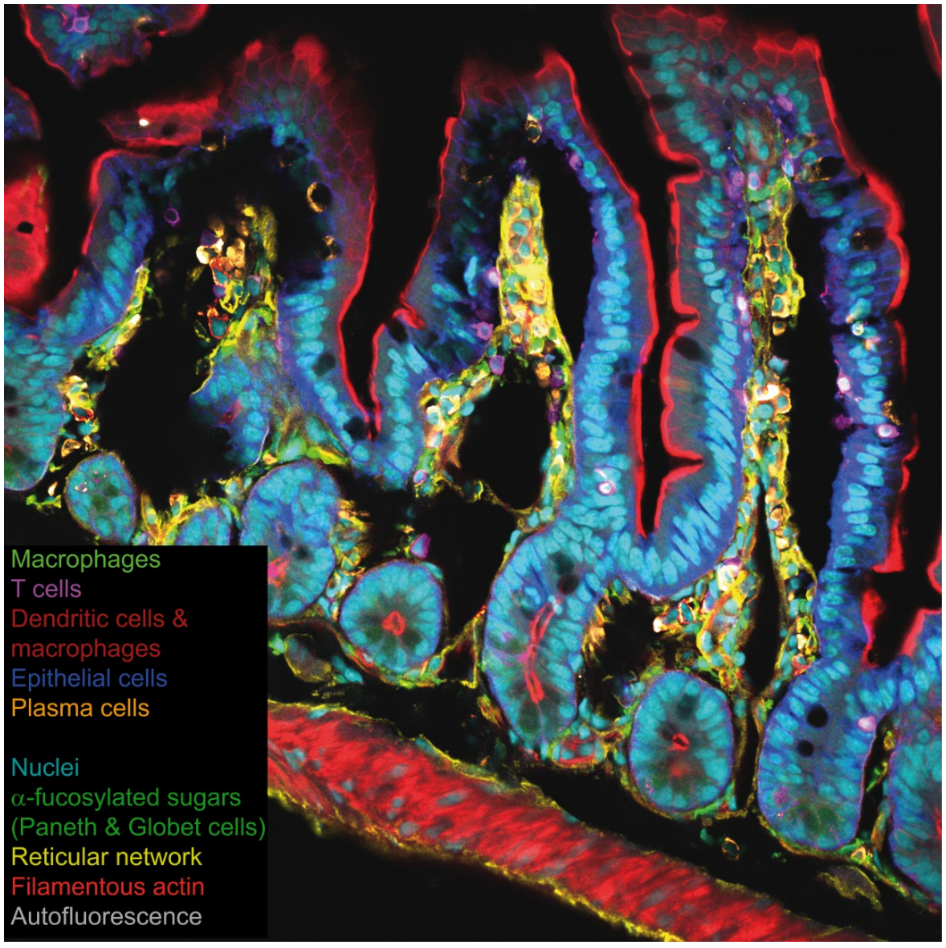
A 10 color confocal spectral imaging strategy to reveal localization of gut immune cell subset. Lelouard, Mailfert & Fallet, Zeiss Application Note, 2018.
Jean-Pierre Gorvel's lab and ImagImm, CIML
Video-microscopy
The video-microscope is a widefield microscope dedicated to long time-lapse experiment and HCS (High Contents Screening) studies, for calcium imaging and visualization of fast events. The Cell Observer microscope is an inverted microscope with an incubation box (temperature and C02 control) and an automatic focus (Definite Focus) to keep the focus during the acquisition. The Zen blue software allows to perform advanced multiple position acquisition on multiple wells or acquire mosaic images.
Microscope available :
Observer Z.1 Zeiss with Hamamatsu ORCA Flash 4.0 LT+ sCMOS camera and 37°C and CO2 chamber, Definite focus.
Phase Imaging
The SID4bio from Phasics is an innovative technique for quantitative phase imaging in light microscopy. Based on a patented technology (the 4-wave lateral shearing interferometry), SID4bio enables observing living cells with no marker, and running statistical and quantitative analysis on them (migration, growth, intracellular processes, etc.).
The SID4Bio provides an unrivalled contrast enhancement of biological specimen without the use of any marker. It enables: studying cell morphology and organelles, time lapse observation over long period of time, reliable post processing such as segmentation and Automatic counting.
By measuring the true phase shift introduced by the specimen, the SID4bio also gives quantitative information about the specimen. It enables: cell dry mass investigation, comparison between cells population, understanding organelles properties based on the optical refractive index assessment.
From http://www.phasicscorp.com
Microscope available :
Phasics SID4BIO on the Zeiss Cell Obsever (see the videomicroscopy part)
Example of results:
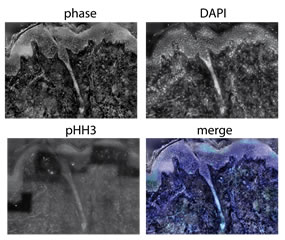
Left| COS7 cells division during 7h imaged by SID4bio (Phase and intensity). Pierre Bon, Hervé Rigneault’s lab, Fresnel Institute / Didier Marguet’s lab, CIML
Right| Mosaïc image reconstruction of a melanoma: phase (SID4bio), nuclei (DAPI), mitotic cells (pHH3).
Marie-Claire Blache, Didier Marguet’s lab, CIML
Intravital and large sample imaging
Multiphoton microscopy
We have developed a 3 colors excitation multiphoton microscope following the work of E. Beaurepaire and colleagues (Mahou et al., Nat Met 2012) called Wavelength mixing. The synchronization of the pump laser with the OPO generates a third wavelength in the focal spot. The non-linear CARS contrast (Coherent Antistokes Raman Scattering) is also available to image lipids in tissue (e.g. myelin).
Microscope available :
Leica SP5 MP with Coherent Ultra II and OPO Chameleon (140fs pulse width)
Example of results :
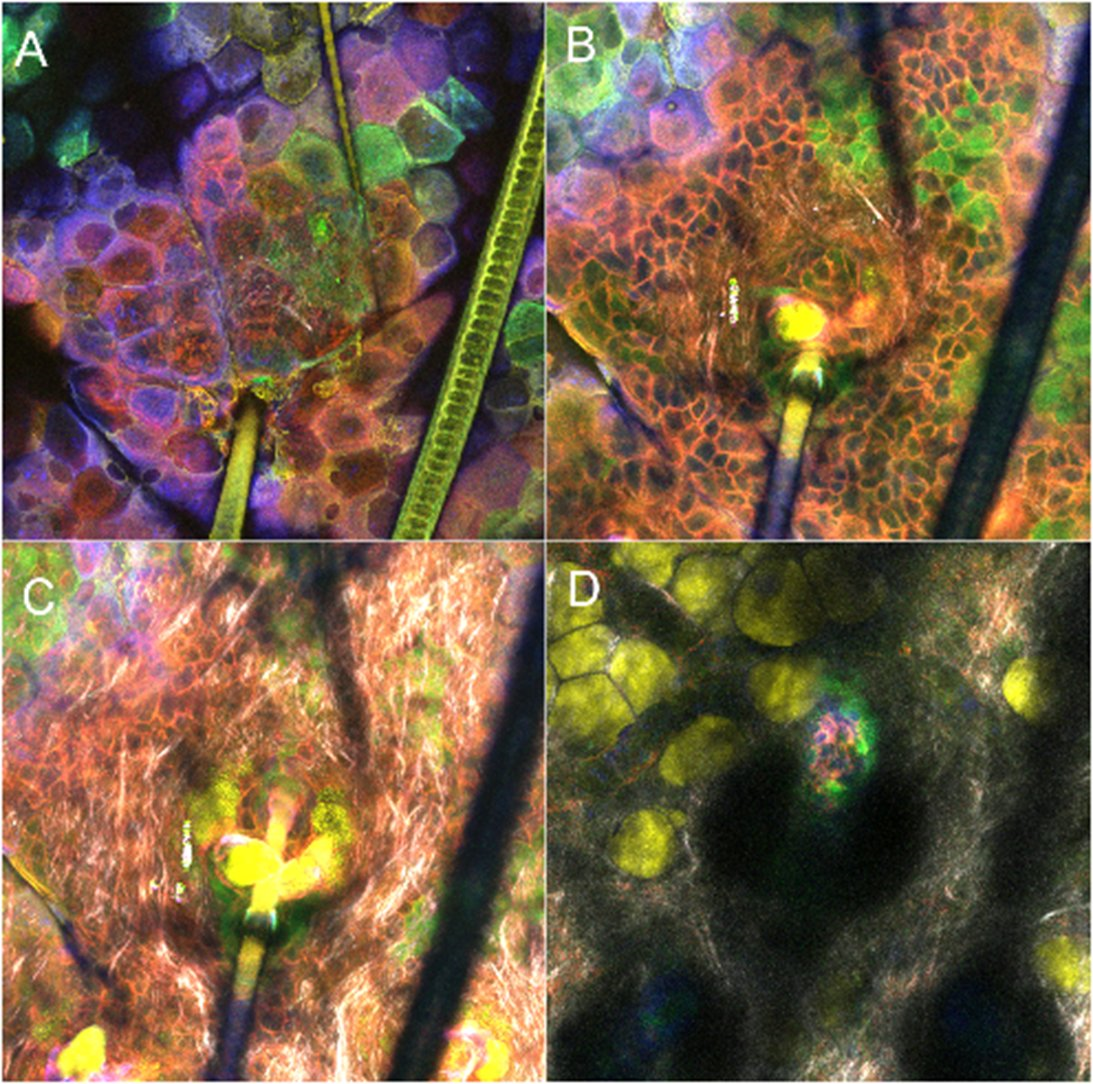
Mouse ear in confetti construct : epidermis (A), dermis (B et C), hypodermis (D).
Confetti (blue/red/green), SHG gray CARS in yellow. Sophie Brustlein (CENTURI)
Light-sheet microscopy
Light-sheet microscopy allows multidimensional imaging with a low photodamage due to the use of a thin sheet of excitation vs. a confined scanning spot in classical confocal microscopy. 3D imaging is performed by scanning the sample with this light-sheet.
The light sheet microscope can image samples ranging in size from few µm up to more than 1 cm. In case of large samples no stitching is required due to a field of view diagonal from 1.7 mm to 17 mm.
Microscope available :
UltraMicroscope II from LaVisionBiotec
Example of results :
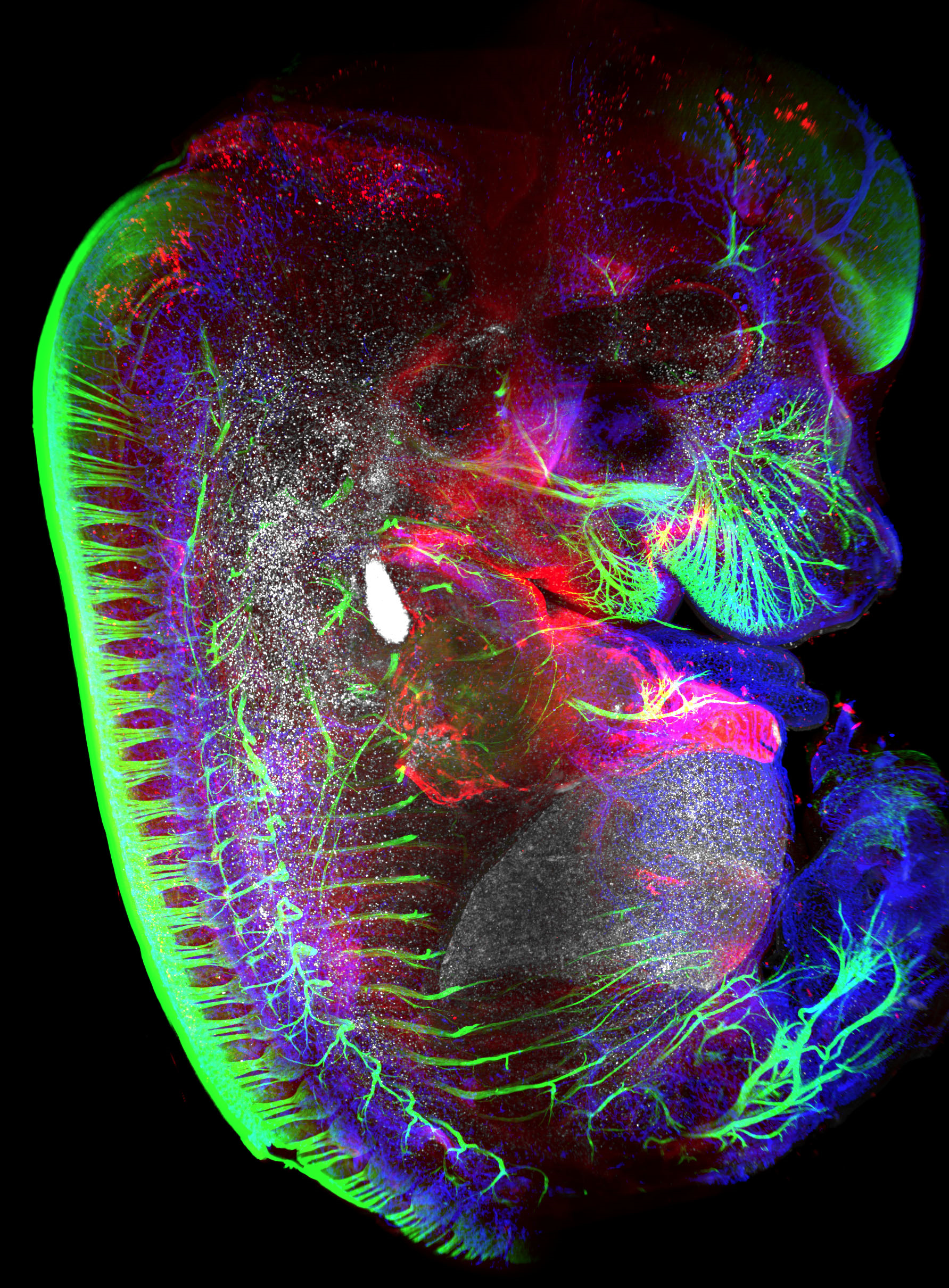
Hematopoietic cells (red), transcription factor Prox-1 (blue), blood vasculature (white) and neurons (green) within a mouse embryo at embryonic day 13.5.
Serge Van de Pavert
Slide scanner
The slide scanner can acquire a tissu in coloration or fluorescence with a 20x/08 or 40x/0.95 magnification. A z stack acqusistion could be obtained to increase the contrast. using a maximum projection. The scanner can load up to 150 slides.
Microscope available :
Pannoramic SCAN II with DAPI/A488/A555/A647 fluorescence cube
Example of results :
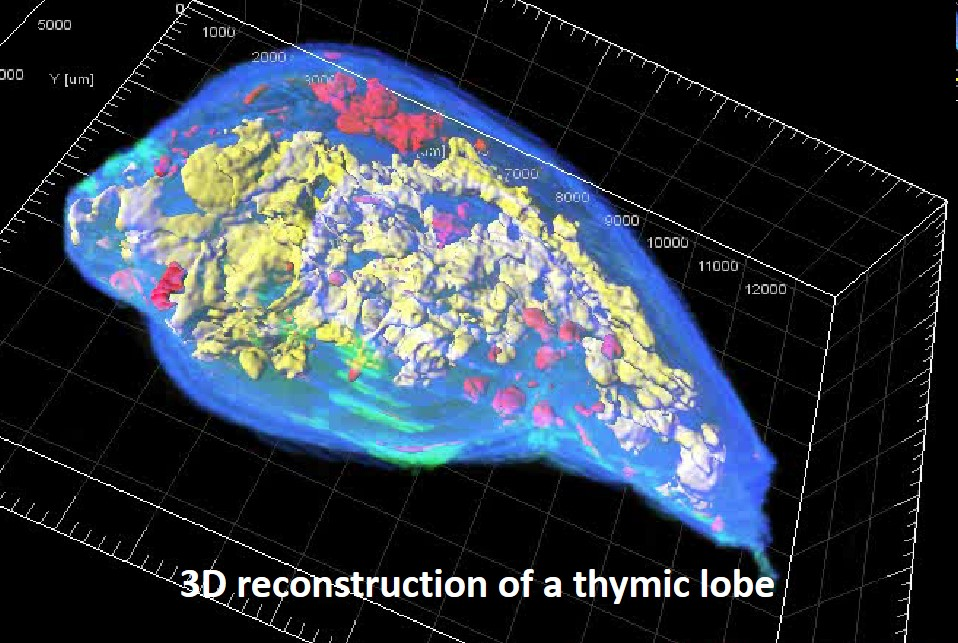
Reconstruction of a thymic lobe from an exhaustive set of serial sections processed by the homemade algorithm For3D.
Each image was acquired with the automated slide scanner. Medullary islets are color encoded according to their volume, from red (smallest) to yellow (largest).
The For3D method is described in Sergé et al. For3D: Full organ reconstruction in 3D, an automatized tool for deciphering the complexity of lymphoid organs. J Immunol Methods. 2015 Sep;424:32-42.
Magali Irla in Philippe Naquet’s lab
Molecular Imaging
Nanoscopy / Single Particle Tracking
Super-resolution microscopy bypasses the optical diffraction limit leading to an improvement up to 20 times compared to classical confocal microscopy. Our setup is fully homemade as we designed the optical path and the acquisition software. Furthermore, we developed an ImageJ plugin dedicated to single molecule localization microscopy (SMLM) called UNLOC (Unsupervised particle LOCalization, see the software tab, Mailfert et al. BioPhys J 2018).
Microscope available :
Nikon Ti Eclipse microscope with Andor Ixon 897 camera, Coherent Obis 405 and Innova-70C lasers
Example of results :
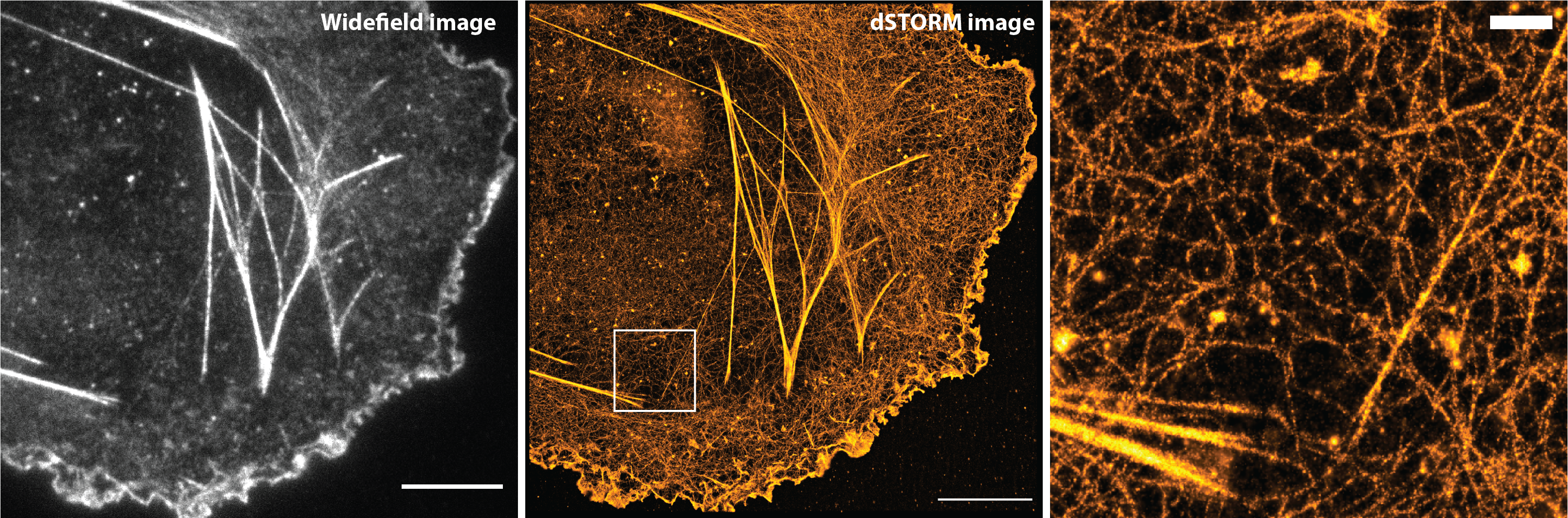
COS7 cells stained for actin with Phalloidin-Alexa Fluor 647. Scale bars: 10 and 1 µm. Images: Roxane Fabre, CIML, Marseille. Analysis software: Sébastien Mailfert – Nicolas Bertaux (He & Marguet’s lab, CIML and PhyTI’s lab, Institut Fresnel – Centrale Marseille)
Fluorescence Correlation Spectroscopy / Fluorescence Recovery After Photobleaching
Fluorescence Correlation Spectroscopy is a powerful technique which allows the measurement of diffusion processes. The specificity of our system is that we can perform FCS at different observation scales (spot variation FCS or svFCS) and thus explore confinement constraints within cell membranes. Spot FRAP experiments are also available on the same homemade setup. A second system is designed to perform Fluorescence Cross-Correlation Spectroscopy (FCCS) in order to study molecular interactions on living cells.
svFCS / spot-FRAP and FCCS systems are designed on Axiovert 200M microscopes with 488 and 633nm lasers, 40X water immersion objectives and SPCMs.
These setups are developed in close collaboration with the Mosaic team from the Fresnel Institute in Marseille.
Microscopes available :
Zeiss Axiovert 200M x2, Coherent Innova-70 and Sapphire lasers, Perkin Elmer SPCMs and incubation boxes
Example of results :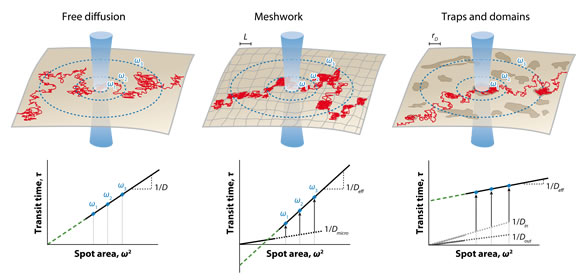
Detecting nanodomains in living cell membrane by fluorescence correlation spectroscopy. He HT, Marguet D. Annu Rev Phys Chem. 2011 May;62:417-36