Our research is located at the interface of immunology and stem cell research. We study the molecular and cellular mechanisms of self-renewal and cell fate choice that guide the differentiation from hematopoietic stem cells to macrophages, cells with important roles in immunity and tissue regeneration.
We are interested in the regulation of these processes by transcription factors, particularly of the Maf family. Currently we develop research projects investigating the lineage commitment of hematopoietic stem cells, self-renewal and reprogramming in mature macrophages and the role of monocyte/macrophage subtypes in inflammation and tissue regeneration.
Overview
A central interest of the laboratory is the development and function of monocytes and macrophages. These cells play critical roles in tissue homeostasis, inflammation and immunity.
We study the mechanisms controlling proliferation and cell fate decisions during monocytic differentiation from hematopoietic stem cells to mature myeloid cells. Specifically we would like to understand the transcriptional regulation of self-renewal and commitment, with particular focus on transcription factors of the Maf family (MafB, c-Maf and MafA).
We develop research projects investigating the lineage commitment of hematopoietic stem cells, self-renewal and reprogramming in mature macrophages and the role of monocyte/macrophage subtypes in inflammation and tissue regeneration.
Lineage commitment of hematopoietic stem cells
We investigate the mechanisms that control self-renewal and differentiation of hematopoietic stem cells (HSC). Resolving a long debate over instructive versus permissive action of cytokines on HSC fate we could show that commitment to the myeloid lineage is controlled by an integrated cytokine/transcription factor circuit, where variation in cell intrinsic sensitivity limits set by a transcription factor such as MafB can render external cytokine cues such as M-CSF instructive (Cell 138: 300; 2009).
Currently we investigate the role of asymmetric divisions and the niche microenvironment on HSC lineage commitment, combining mouse genetics and in vivo analysis with cell culture models and video-imaging.
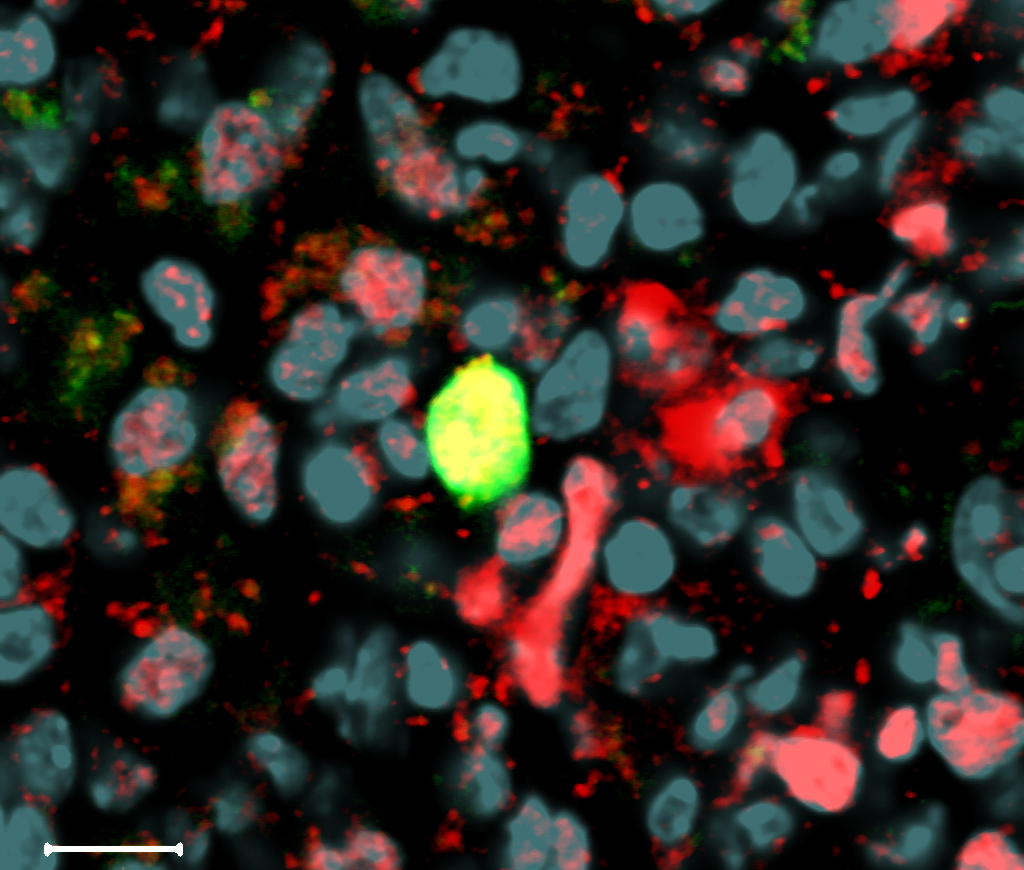
A myeloid committed HSC in the spleen. Copyright S Sarrazin, M Sieweke, CIML
Self-renewal and reprogramming of differentiated cells
Terminal differentiation is typically tightly linked to cell cycle withdrawal. However, we discovered that MafB/cMaf deficiency enables self-renewal of differentiated functional macrophages by a cMyc/KLF4 dependent mechanism. It thus appears possible to amplify functional differentiated cells without malignant transformation or stem cell intermediates (Science 326:867 (2009)). We currently investigate the mechanisms of self-renewal in differentiated cells and whether it facilitates direct reprogramming. We also explore the therapeutic potential of amplified macrophages and their utility as a research tool.
Macrophages in tissue regeneration
Inflammation and tissue regeneration are often linked processes but require precise timing and coordination of different cell types. In this context we investigate the cell fate decisions that give rise to different monocyte subtypes and their macrophage or dendritic cell descendents. We want to identify the transcription factors controlling these choices and aim to understand how their manipulation affects tissue repair.