My team focuses on dendritic cell (DC) subsets as a link between innate and adaptive immunity, sensing/responding to infections early while orchestrating downstream T cell responses.
We are trying to unravel the aspects of DC subset functional specialization and regulation that are conserved between higher vertebrates. We are making a major effort to accelerate knowledge translation from mouse to humans to help accelerate clinical applications of fundamental discoveries initially made in mice
Overview
Harnessing comparative genomics as a tool to predict human DC subset functions
The development of better vaccines or immunotherapies against cancer or intracellular pathogens is one of the major current challenges of immunological research. Innovative approaches are needed to induce efficient and long-lasting memory cytotoxic CD8 T cell (CTL) responses.
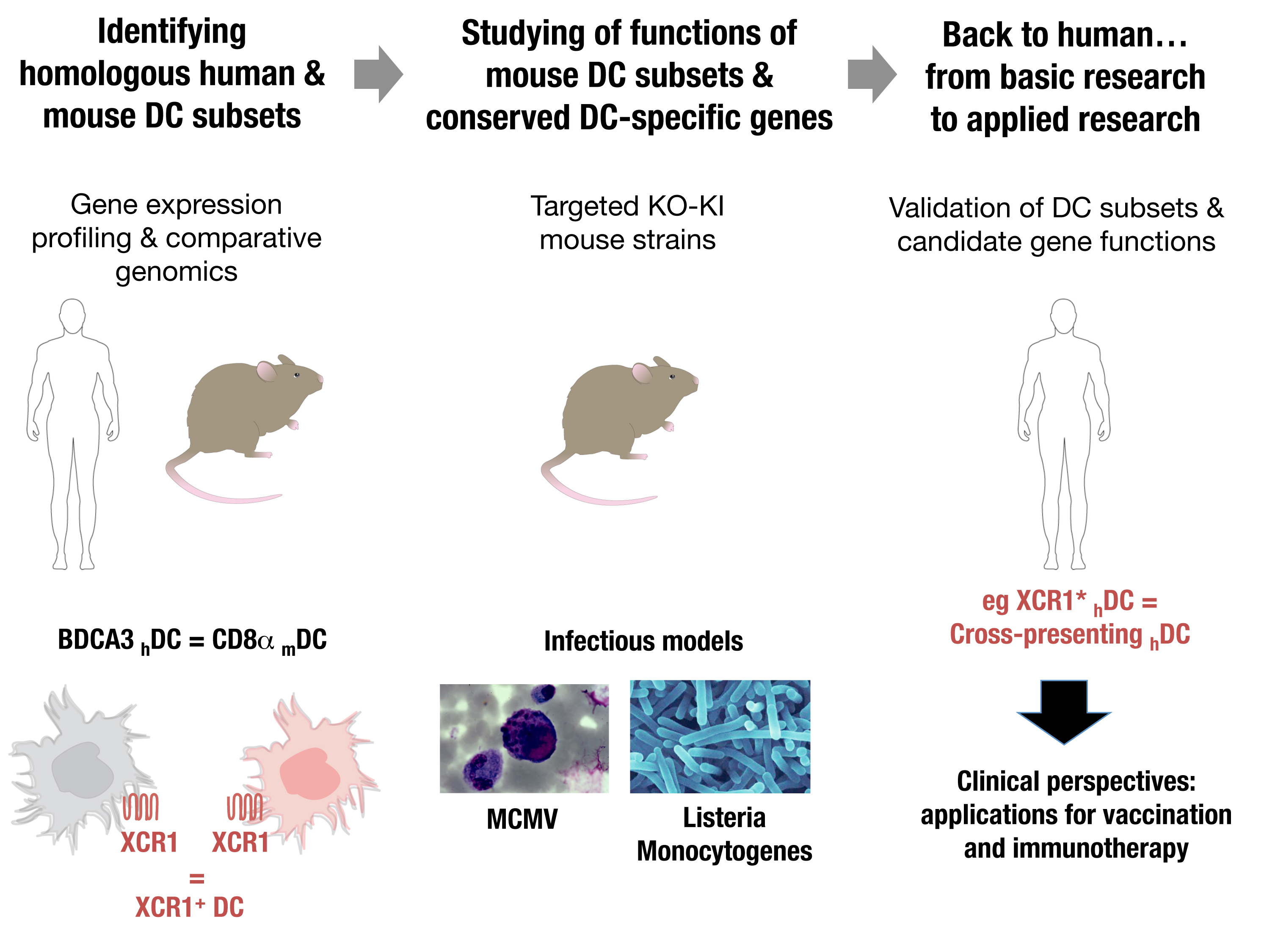
A particular subset of mouse dendritic cells (DC) is specialized in this function: the CD8α mouse DC, which excels at antigen cross-presentation. Their targeting in vivo for vaccination, by co-administration of adjuvants and antigen-fused specific antibodies, yielded very encouraging results.
However, the efficacy of the immune responses induced and the generation of protective long-term memory require improvement. Moreover, how to translate this vaccination strategy for human health was not obvious, because no human DC subset equivalent to CD8α mouse DC had been discovered. Investigating the relationships between mouse and human DC subsets was hampered by the use of different markers for their identification.
To overcome this cognitive gap, we recently developed an original strategy. We harnessed comparative genomics as a tool to unravel potential functional equivalences between mouse DC human DC subsets based on the similarities of their gene expression programs. This led us to propose, and to confirm by functional studies, that BDCA3 human DC are professional cross-presenting DC equivalent to CD8α mouse DC.
However, we still do not know what the extent of the physiological functions of different DC subsets is, and we largely ignore how they are regulated.
 Harnessing comparative genomics to advance knowledge on DC subset biology
Harnessing comparative genomics to advance knowledge on DC subset biology
Our basic hypothesis is that a significant homology exists between the DC subset from human (hDC) and mouse (mDC). This is supported by our published work demonstrating that BDCA3 hDC share a specific gene signature with CD8α mDC and are professional cross presenting hDC. Hence, focusing on the functions of genes/pathways with a conserved selective expression between equivalent human and mouse DC subsets will likely unravel novel critical aspects of the functions of these cells and their molecular regulation, with the potential for rapid translation of this knowledge into clinical use
A systems biology approach to unravel mouse and human DC subset functions and regulation
We are developing a major, innovative and unprecedented effort to investigate in parallel in mouse and human the biology of two DC subsets thought to be important for antiviral and antitumoral defense: plasmacytoid DC and professional cross-presenting DC. We designed a Systems Biology approach to uncover key functions and regulatory pathways conserved in DC subsets through evolution. We will investigate the extent of DC subset functions and their molecular regulation in vivo during infectious challenges in mice, by using state-of-the-art technology to generate and analyze novel high throughput data and innovative mutant mouse strains.
We will focus some of our investigations on candidate genes/pathways which we have identified through our comparative genomics analyses as selectively expressed and/or differentially acting in BDCA3 human DC and CD8α mouse DC as compared to other leukocytes. These candidate genes must likely play key roles in the regulation of the functions of BDCA3 human DC and CD8α mouse DC. We will validate the relevance for the human of the knowledge obtained in the mouse, through comparative genomics and in vitro experiments on human DC subsets.
This approach will yield novel knowledge to understand the pivotal role of DC subsets in the functioning of the immune system, and its regulation, with a high potential for rapid translation into clinical use.