Geolocation and functions of a new family of lymphocytes
In mammals, the innate immune system works using a wide range of specialized cells: dendritic cells, polymorphonuclear leukocytes, monocytes, macrophages, granulocytes and NK cells. Together, they comprise an amazingly effective rapid defense system and trigger the adaptive immune response.
ILCs (Innate Lymphoid Cells) are the last described members of the family. They are somehow a “fast version” of T lymphocytes. Distributed in various groups, they have the morphology of a lymphoid cell, produce the same cocktails of cytokines as T lymphocytes but, just like their innate immune congeners, they lack antigen-specific receptors. Serge van de Pavert and his team are particularly interested in the third subgroup of ILCs, ILC3, which is involved in the development of secondary lymphoid organs. By hosting the antigen presentation from invading pathogens to mature lymphocytes, these peripherical organs play a key role in the adaptative immune system.
From a functional point of view, ILCs can be classified into two categories: natural killer (NK) cells and the so-called auxiliary ILCs which relay activation signals to other immune cells. Depending on the molecules they express (surface markers, transcription factors and cytokines), ILCs can be divided into three subgroups: ILC1s which express transcription factor T-bet and produce the IFNγ cytokine, ILC2s which express transcription factor GATA-3 and produce the same cytokines as type-2 T helper cells and, lastly, ILC3s whose development and function depend on transcription factor RORγt.
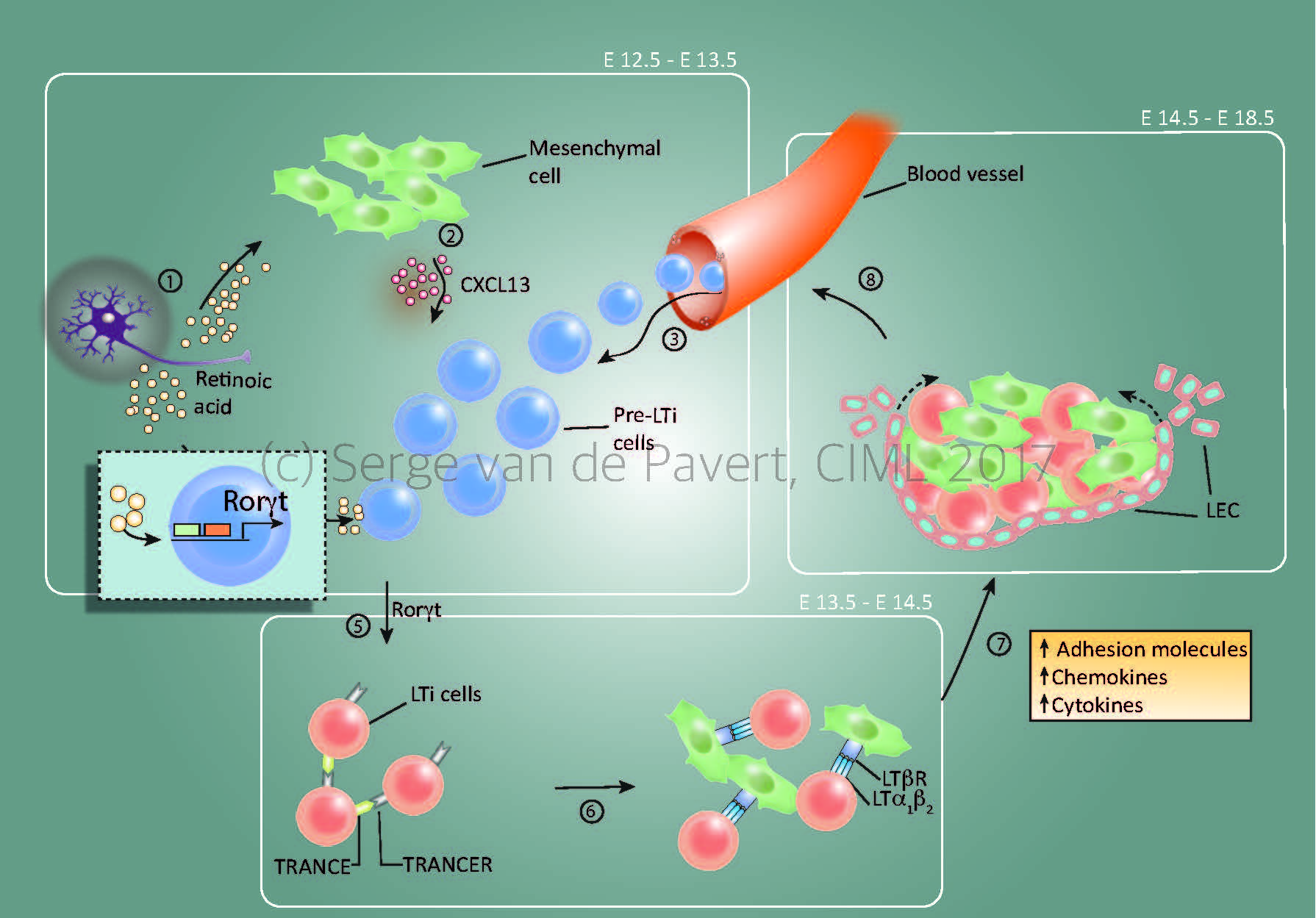
“Lymph node formation within the mouse embryo starts around E12.5 by Cxcl13 secretion by resident mesenchymal cells, under influence by retinoic acid. The stage at which the lymph node anlagen is initiated depends on the location within the embryo, with the most anterior lymph nodes (E12.5= cervical, mandibular, axillary) forming the first and the most posterior as last (E14.5/15.5= inguinal, popliteal). The presence of Cxcl13 and possibly other chemokines expressed by the mesenchymal cells, attract (pre-) Lymphoid Tissue Inducer (LTi) cells towards the niche. Within this niche, retinoic acid induces the essential nuclear transcription factor RORγt within the pre-LTi cells so that they mature into LTi cells, which can interact with the mesenchymal/stromal organiser cells via the lymphotoxin signalling pathway.
Expansion of the lymph node anlagen takes place after lymphotoxin signalling started (around E14.5 for the most anterior lymph nodes), with the attraction of more LTi cells, and thus more interactions with resident mesenchymal cells. Also, the mesenchymal cells produce Vegfc, a potent attraction molecule for lymphatic endothelial cells, which form a layer of lymphatic endothelial cells around the lymph node anlagen and eventually connect it to the lymphatic vasculature. At this stage, the chemokine Ccl21 expressed by the lymphatic endothelial cells and vasculature are important for the transport of LTi cells towards the lymph node anlagen.”
The adaptive immune system: a protective teamwork
Lymphatic vessels and lymph nodes are two key components of our defense against pathogens. The first ones transport cells and small molecules derived from peripheral sites to the second ones where the adaptive immune response is organized. The proper location of these two components is crucial to trigger an adequate and fast immune response. It is therefore not surprising that both lymphatic vessels and lymph nodes are formed in a coordinated fashion, as lymphatic vessels and lymph nodes appear within the same time frame, while sharing essential differentiation signals.
Lymph nodes are formed during embryogenesis, and the first steps in this process involve the interaction of mesenchymal organizer cells with ILC3s at specific locations throughout the body. Although processes leading to embryonic lymph node formation and lymphatic vessels development are well known, it is still unclear how they are initiated at specific locations. “Based on our results and preliminary data, nerve fibers and blood vessel bifurcations could play a role” commented Serge van de Pavert. “The overall objective of my current research is to characterize all players involved in development of the immune system, specifically in relation to the formation of lymph nodes and lymphatic vessels.”
“You are what your mother ate”
Secondary lymphoid organ formation depends on ILC3s, also called lymphoid tissue inducer (LTi) cells. Within the group of prof. Reina Mebius, Serge van de Pavert and his colleagues have shown that mouse fetal ILC3s are controlled by retinoic acid (RA), the metabolic product of vitamin A. RA works on preparing ILC progenitors to interact with each other, and changing the amount of RA has severe effect on their behavior. Consistently, do mothers fed a vitamin A-deficient diet give birth to children with reduced immune capacities? “This appears to be the case” Serge van de Pavert pointed out “We have observed that mothers supplied with a vitamin A-deficient diet during pregnancy, give birth to offspring with small lymph nodes. And smaller lymph nodes lead to a lowered immune response”. This observation suggests that, above and beyond the physical disabilities produced by malnutrition in mothers and children, the latter may also be unable to develop effective immune response.
Neurons could be involved during formation of lymph node
“Lymph nodes always develop in the same locations near large veins and at the junctions of blood vessels. So there is every reason to believe that these sites emit signals that lead to the development of these organs. Recent data back up the hypothesis that retinoic acid is produced directly by the neurons present in the surrounding environment, which demonstrates the close connection between the nervous and immune systems.”
The team is now seeking to determine the role of ILC3s within this environment, which notably requires perfecting the phenotyping of the various ILC3s subpopulations. While researchers have managed to establish the main ILCs lines, they still do not have a precise map indicating both their different stages of differentiation and the many interactions that they establish with their environments. “Our objective is to understand how ILC3 respond and adapt to their micro-environment.” Alongside this functional analysis we can mention a descriptive study aimed at mapping ILC3s’ environment using LSM (Light Sheet Fluorescence Microscopy). This state-of-the-art technology is able to generate quick and high resolution 3D analysis of large tissues. The 3D data obtained can thus be used to monitor the interactions that lymph nodes have with their environment in real time, notably with nerve cells and blood vessels.
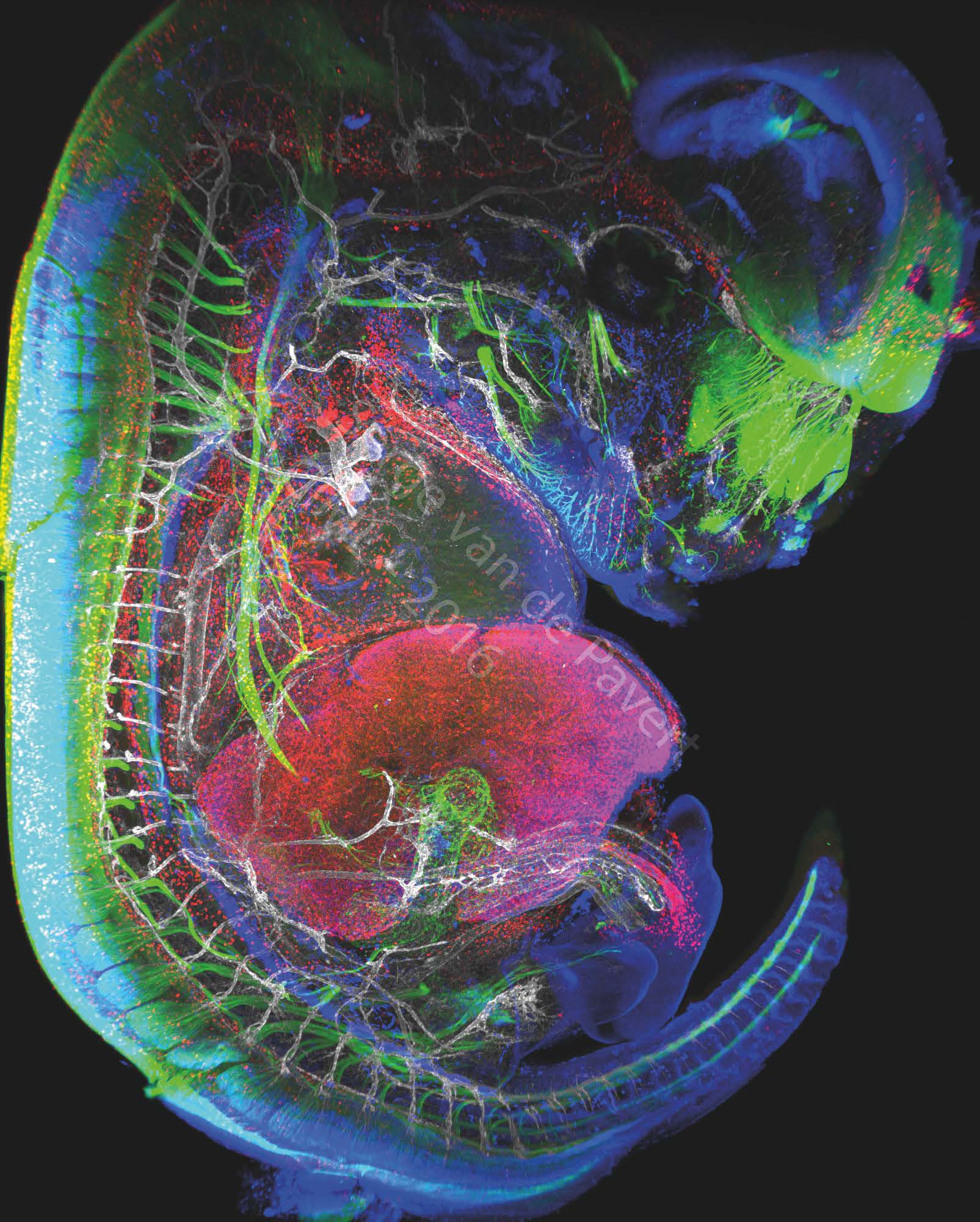
"Immunofluorescent staining of a complete mouse E13.5 embryo reveals neurons (labeled with BetaIII Tubulin), macrophages and lymphatic endothelial cells in red (labeled with Lyve-1), the nuclei of specific neurons and lymphatic endothelial cells are marked in blue (Prox1 labeling) and the vascular network in white (Pecam-1 labeling). The eye of the embryo and the nasal neurons can be seen in green. On the left, the spinal cord is marked in green. The vertebrae are visible through the marking of the dorsal root ganglia in green, and blood vessels marked in white. The heart is visible just above the liver which is labeled with LYVE-1 antibody. The embryonic intestine, not far from the liver, is visualized by the innervation of neurons in green. The umbilical cord can be observed by neighboring white blood vessels and macrophages in red. This picture was generated in the study on the formation of lymph nodes and lymphatic system. Their formation, initiated in the embryonic stage, can be modified by changing certain dietary factors affecting cell differentiation. These visualizations are therefore intended to analyze the movements of cells involved in the formation of the lymphatic system.” Image Serge van de Pavert/CIML