Stromal cells
Stromal cells are connective tissue cells present in all our organs. Within lymphoid organs such as lymph nodes, they constitute a migrating and nurturing scaffold for leukocytes. Within non-lymphoid organs, they are best known to support the function of parenchymal cells. However, growing evidence suggest that stromal cells also control the homeostasis of tissue-resident immune cells such as macrophages.
Our laboratory is studying (1) the immune-regulatory functions of stromal cells in lymphoid organs and (2) the stromal cell-Macrophage crosstalk in non lymphoid organs.
AIM I
Immunobiology of stromal cells in lymphoid organs
“A readership consisting of primarily anatomists has every right to question the favorite sport of research workers in cell immunology. This is to take a lymphoid tissue and totally destroy its beautiful and elaborately designed architecture to obtain simple cell suspension of lymphocytes, which are then asked to do more or less all the jobs of the original anatomic masterpiece’’ Nossal, 1984.
Functions of LN stromal cells. Lymph nodes (LNs) are composed of leukocytes (~95%) and lymphoid stromal cells (~5%) that form the structural framework of these organs. Stromal cells have been considered for decades as inert elements of the immune system but this view has dramatically changed in recent years, when it was discovered that they are endowed with immuno-regulatory functions. Within lymphoid organs, various stromal cell subsets create dense three-dimensional (3D) cellular networks. These control lymphocyte survival and migration, create the backbone of the LN and provide the nutrients, soluble factors, antigens and the distinct immune cells required for ‘immunological surveillance’ and the development of adaptive immune responses. Indeed, immune cells would not properly function or even survive without these stromal cell networks. Thus, a better understanding of their biology is mandatory to our full comprehension of the immune system.
Dynamics of LN stromal cells. In mammals, LNs develop during embryogenesis in a series of subtle cellular interactions. Early after birth, leukocytes colonize the LNs and orchestrate their organization. While being very complex, the structuration of these stromal cell networks is not definitive. Upon immunization, LNs undergo a transient but tremendous enlargement that is supported by an extensive remodelling of the various stromal cell networks and the generation of new microenvironments necessary for the development of the immune response (e.g Germinal Centers, medullary cords). So far, the mechanisms governing the spatio-temporal development and inflammation-induced remodeling of the different LN stromal cell susbets as well as their putative precursor-product relationship are poorly understood. This lack of knowledge results from technical challenges inherent to the isolation and culture of stromal cells, the inability to model the complexity of LN organization in vitro and the lack of animal models dedicated to their study. Our stromal cell research is focused on investigating the origin and dynamics of LN stromal cell types at steady state and under inflammatory conditions. Our aim is to provide the first “Phylogenetic tree” of LN stroma development and remodeling at the single cell level resolution. As this challenging goal cannot be reached by in vitro/ex vivo techniques, we are developing cutting-edge mouse models that allow us to map the fate of single LN stromal cells in situ, in their natural microenvironment (Figure 1).
A B
C D
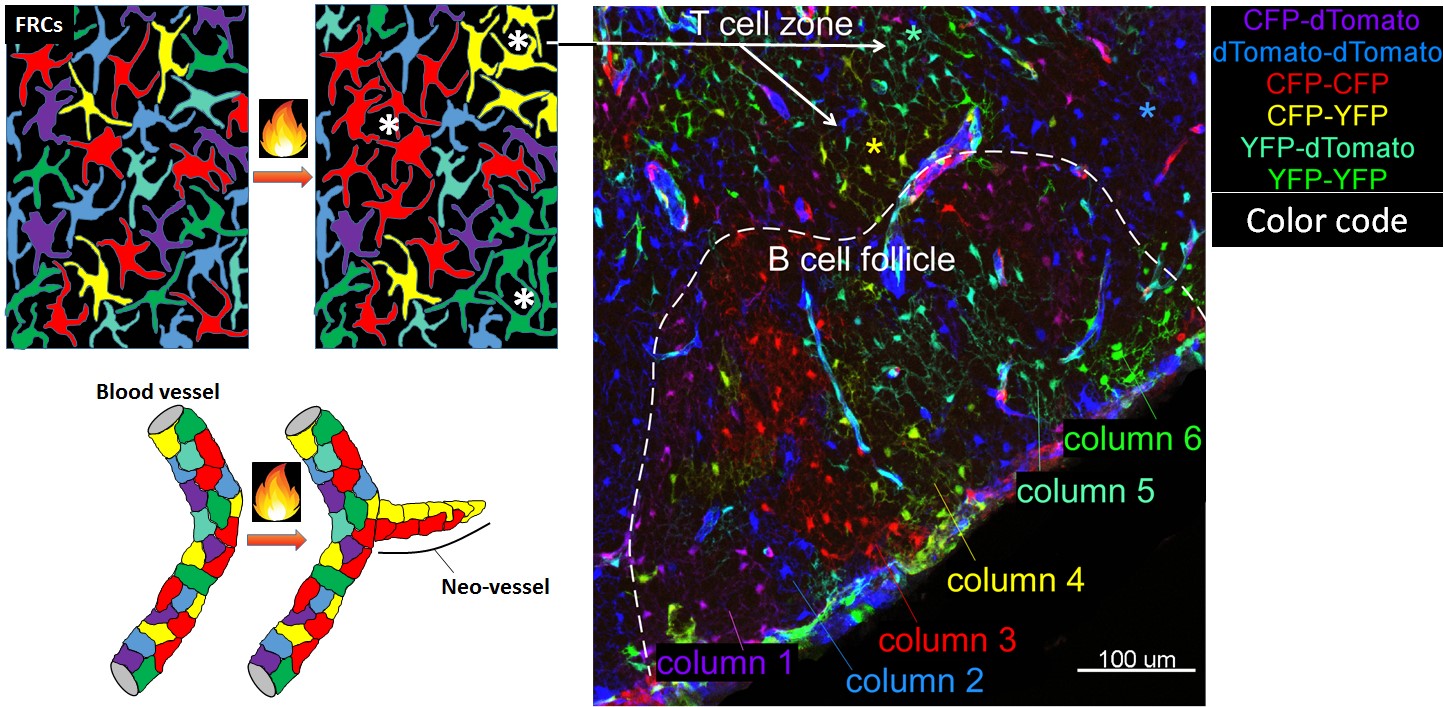
Figure 1: Dynamics of lymphoid stromal cells
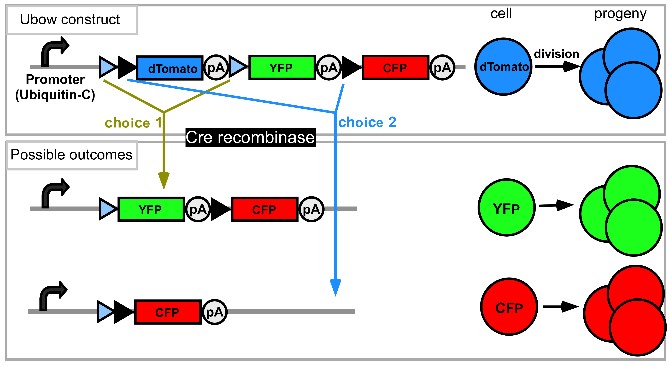
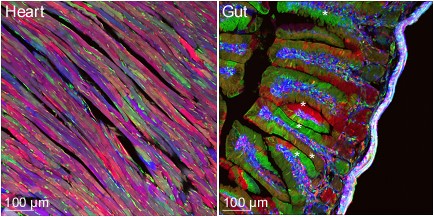
(A) In the Ubow construct, incompatible sets of lox sites (blue and black triangles) alternate: Cre chooses between excision events 1 or 2. Before Cre action, only the gene following the promoter is expressed (dTomato). Recombination switches expression to either CFP (1st possibility) or YFP (2nd possibility). (B) Mice homozygous for the Ubow construct (and thus able to express 6 distinct colors) were crossed to inducible Ubiquitin-CreERT2 mice. Adult mice were treated with tamoxifen in order to trigger Cre recombinase in all the cells of the mouse. Various organs were sectioned and imaged by confocal microscopy 1 month after the end of the tamoxifen treatment. Note the mono-colored columns of rapidly renewing enterocytes in the gut (*) indicative of successful fate mapping. (C) Examples of possible proliferative behavior of LN fibroblasts and blood endothelial cells upon inflammation in a Ubow mouse. * indicates foci of fibroblast proliferation revealed by monocolored clusters of fibroblasts. (D) Utility of the Ubow mouse to study stromal cell biology. Ubow++ Ubiquitin CreERT2 mice were irradiated and reconstituted with Wt bone marrow cells. Reconstituted chimeras were treated with Tamoxifen in order to trigger the stochastic recombination of colors in radio-resistant stromal cells. Mice were injected with adjuvant in their footpads and draining LNs were imaged 3 weeks later by confocal microscopy. Note the presence of monocolored clusters of fibroblasts (*) in the T cell zone and their organization in monocolored columns in B cell follicle. No clusters/columns were observed in non-inflamed LNs (not shown).
AIM II
Stromal cell-Macrophage crosstalk in non-lymphoid organs
“Macrophages can be thought of as a dispersed homeostatic organ” Gordon, 2017.
The concept of the macrophage niche. All organs in the body require and contain macrophages (Mϕ), which frequently take on specialized properties to support trophic functions specific to their tissue of residence. Mϕ themselves depend on environmental cues such as CX3CL1, IL34, Colony Stimulating Factor (CSF)1 and CSF2 for their development and maintenance. These molecules are thought to be derived from “Mϕ niches” within tissues, which proposedly (i) provide a 3D anchoring scaffold for Mϕ and (ii) nurture them by cytokine production. In return, Mϕ should provide positive “feedback” signals to their niche, generating two-cell circuits benefiting each partner (Figure 2). The nature of these niches and their role in the regulation of Mϕ homeostasis remains to be investigated.
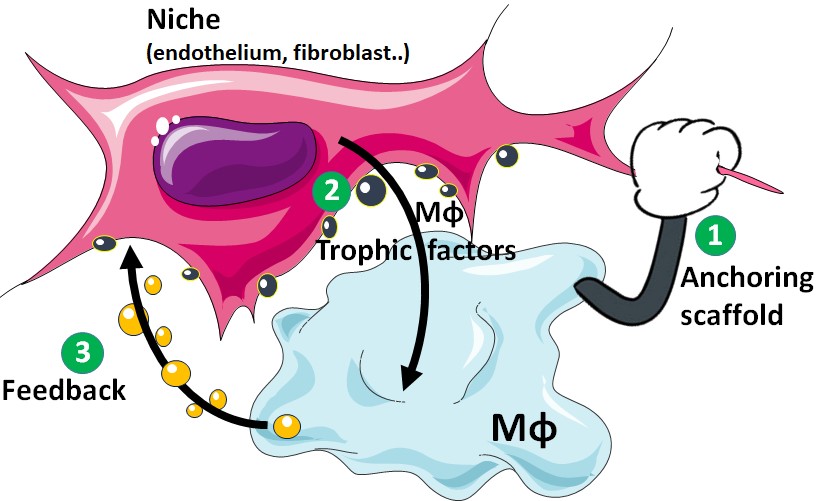
Figure 2: The concept of the Mϕ niche
1- The Mϕ niche provides an anchoring scaffold to its Mϕ.
2- The Mϕ niche produces membrane bound Mϕ survival factors, ensuring a close proximity between the 2 partners.
3- Mϕ produce a positive feedback signal to its niche, creating a two-cell circuit benefiting each partner.
Stromal cells as Mϕ niches. Just like Mϕ, stromal cells such as fibroblasts, epithelial and endothelial cells are abundant, sessile cells that are ubiquitously distributed throughout our organism. Within lymphoid organs, stromal cells act as niches for T and B cells via the provision of survival factors and their ability to create 3D cellular networks on which lymphocytes actively migrate. Importantly, we were among the first to demonstrate that stromal cells also regulate Mϕ homeostasis via the production of CSF1 in LN and spleen, providing the first proof of principle that stromal cells can represent an essential component of the Mϕ niche in lymphoid organs. We have now gathered solid evidence that this phenomenon is not an exception but rather, the rule across tissues. Our research in this area is focused on (i) establishing the first integrative atlas of Mϕ niches across tissues, (ii) deciphering the molecular mechanisms underlying stroma- Mϕ crosstalk and (iii) unravelling the biological functions of Mϕ-stroma cell circuits.