Understanding the mechanisms of T-cell tolerance to develop innovative strategies to prevent and treat autoimmune diseases.
The immune system has the remarkable ability to discriminate between self and non-self. The lack of immune response towards our tissues is called "immunological tolerance". This essential process prevents potential immune attacks on our tissues. Dysregulation leads to the development of autoimmune pathologies that affect 5 to 10% of the population. The thymus plays an essential role in the establishment of T lymphocyte tolerance, major actors of the immune system through their ability to fight infections and tumors.
Magali Irla and her team aim to characterize the cellular actors of immune tolerance and to understand the underlying molecular mechanisms in order to identify new therapeutic strategies for autoimmune diseases and the regeneration of the immune system.
The thymus is a primary lymphoid organ, which coordinates the developmental processes leading to the generation of functional and non-autoreactive T lymphocytes. The establishment of T-cell tolerance in this organ is characterized by two major processes:
- The elimination of autoreactive T cells that recognize self-antigens. This process is called clonal deletion or negative selection.
- The generation of regulatory T cells (Tregs) that possess the unique ability to control autoreactive T cells that have escaped thymic selection. Tregs thus play an essential role in maintaining self-tolerance in the periphery.
Both processes occur primarily in the thymic medulla, which is composed of a dense network of medullary thymic epithelial cells (mTECs) and dendritic cells (DCs). mTECs play a key role in T-cell selection through their fascinating ability to express thousands of peripheral tissue-restricted self-antigens (called auto-antigens). The expression of auto-antigens is regulated notably by Aire and Fezf2 transcription factors.
DCs also participate in the establishment of T-cell tolerance by presenting auto-antigens expressed by mTECs and by transporting into the thymus harmless antigens captured in the periphery. Thus, mTECs and DCs tightly collaborate to generate functional non-autoreactive T lymphocytes and to produce Treg cells.
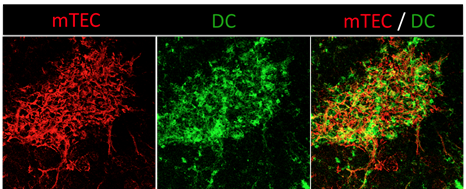
The thymic medulla, site of T-cell tolerance, is composed of a dense network of mTECs and DCs. Confocal microscopy image of a thymic section showing mTECs (anti-keratin 14, red) and DCs (anti-CD11c, green). Copyright: Magali Irla, CIML.
Reciprocally, developing T cells, called thymocytes, control the differentiation and organization of mTECs. This bi-directional phenomenon is called "thymic crosstalk". In this context, the team has shown that CD4+ thymocytes play a key role not only in the development of functional mTECs expressing the transcription factor Aire but also in the 3D organization of the thymic medulla.
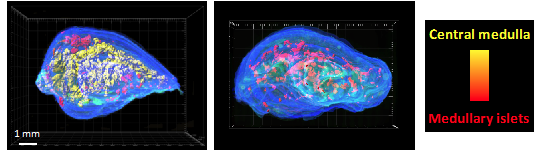
CD4+ thymocytes control the 3D organization of the thymic medulla. 3D reconstruction of thymic lobes from wild-type mice (left image) and H2A-a-/- mice lacking CD4+ thymocytes (right image) using our algorithm For3D (Full organ reconstruction in 3D). The medullary islets are color coded according to their volume, from red (smallest) to yellow (largest). Copyright: Irla et al. Journal of Immunology 2013.
The team currently aims at better understanding the impact of thymocytes on the development of different mTEC subtypes and the expression of auto-antigens in a perspective of regenerative medicine. Since Tregs are highly promising in cell therapy in the context of autoimmunity and transplantation, we are studying their development in the thymus and are seeking to identify molecules that control their suppressive and anti-inflammatory activities in order to facilitate their use in clinic. For this, we use several preclinical mouse models of autoimmunity and transplantation.
Regeneration of the thymic function
The thymic function is severely impaired by myeloablative regimen commonly used to treat hematological diseases by hematopoietic stem cell transplantation. Furthermore, during aging, this organ undergoes progressive changes in its architecture and composition, a phenomenon known as "thymic involution". Myeloablative regimen and aging have deleterious effects on TECs, leading in a reduced production of T lymphocytes and consequently to an increased susceptibility to opportunistic infections, autoimmunity and cancer.
It is therefore essential to identify molecules capable to regenerate the thymus, which shows a high plasticity. Our team is seeking to identify new therapeutic strategies to stimulate thymus regeneration and T-cell production in order to improve immunity in many physio-pathological conditions.