An innate sense of danger
The Toll-like receptor (TLR) family in human is composed of ten receptors, numbered from 1 to 10. Their common function, which has been remarkably conserved over the course of evolution, is to detect signals indicating that an infection is occurring. The TLRs are used by immune cells resident principally in the skin and mucus membranes, the natural sites of entry of pathogens.
To deal with the menace of highly varied infectious organisms, the TLR present a large number of structures in different locations. Once stimulated by signals from microbes these receptors send signals through different pathways to the nucleus of the cell where they activate the immune response, leading to inflammation.
Lena Alexopoulou has been studying the mechanisms of protection against infection that are stimulated by the TLR. She has contributed to the identification of the signals that are recognized by a certain number of these, their origin (viral or bacterial) and their role in protection against different diseases.
Today, her team focuses on two aspects of the biology of TLRs: their implication in autoimmune diseases and the mechanisms of cooperation between different members of this family.
Microbial signals detected by TLRs are very similar to those released by our own cells when they are damaged or dying. Thus, infections are sometimes associated with heightened reactions against cells of the body, according to a mechanism called autoimmunity.
Autoimmunity, side effect of (dis) functioning of TLRs
In an autoimmune disease, the immune system reacts against the organism that it is supposed to protect: components of the self are abnormally identified as foreign by the immune system, and has a result the immune system attack normal cells of the individual. Autoantibodies are found in the blood of patients, signatures of this deleterious self-reactivity.
Recent studies have demonstrated the role of the activation of TLRs in the genesis of some autoimmune diseases, including systemic lupus erythematosus (SLE). SLE is a chronic autoimmune disease characterized by massive production of autoantibodies directed against components of the cell nucleus, leading to skin disorders, and joint and vascular visceral in its worst forms.
"“TLR8-deficient mice develop lupus, because the loss of the TLR8 gene leads to an increase of TLR7 expression and signalling which appears to induce an increased sensitivity to certain compounds," says Lena Alexopoulou.
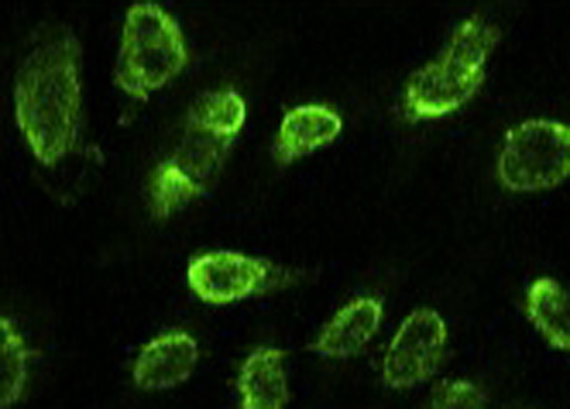
TLR8-deficient mice develop Lupus (detection of antibodies directed against components of the cell nucleus by immunofluorescence).
Copyright Lena Axelopoulou, CIML
Thus, it would be interesting to elucidate the role of TLR8 in SLE and in particular whether there is a correlation between patients with lupus and impairment in the function of TLR8. Physicians could detect and measure this anomaly to refine their diagnosis and assess the prognosis of the disease.
TLR cooperation is important for optimal protection
To move around their environment, bacteria use structures like whips named flagella, composed mainly of the monomer flagellin. The recognition of flagellin by TLR5 signals the presence of pathogenic bacteria and in turn triggers an immune response that protects us against potentially serious bacterial infections.
Nevertheless, mice lacking TLR5 are not particularly susceptible to infection, unless they are also deficient in TLR4, which recognizes a different bacterial compound. Thus, the cooperation between different TLRs is sometimes pivotal for optimal protection against infection.
These two different approaches to the study of the biology of TLRs illustrate the complexity of the innate system of protection against infection:
- The adverse effects related to the loss of control of its activity.
- Its ability to integrate various signals received simultaneously to yield an optimal immune response.