Transactional analysis of the relationships in the couple immune cell-bacteria
Since he was not a microbiologist or immunologist, it took a good deal of scientific courage for Jean-Pierre Gorvel to launch himself, 18 years ago, into the study of interactions between pathogenic bacteria and the cells they infect. His expertise has been built on the collaborations he established with research teams from the four corners of the globe: from Spain to Saudi Arabia, through South Africa, Texas and ...the CIML! Driven by a single credo: apply these discoveries to human health.
Today the team of Jean Pierre Gorvel is trying to understand all facets of this theme: the identification of virulence factors of bacteria responsible for serious disease in humans, optimization of anti-infective vaccines, decryption of the mechanisms used by bacteria escape the vigilance of the immune system, the study of the immune response to infection in the lining of the stomach, lung...
"Our research projects are in synergy with each other, constantly maintained by new ideas, by the acquisition of new technologies and by meeting new colleagues and partners," says Jean-Pierre Gorvel, who follows-up with one of the subjects that is dear to his heart: the bacteria Brucella family. "Right now, we are exploiting the fruits of our discoveries Brucella and the immune response it induces, to the study of its pathogenicity during pregnancy. " Indeed, Brucella, which is endemic in the Mediterranean basin and transmitted to man by cattle, is responsible for abortions, among other mammals and a severe fever called "Malta fever".
Surviving inside of its enemy
During infection, Brucella is absorbed by macrophages, large white blood cells responsible for ridding the body of harmful agents. However, instead of being destroyed by the latter, the bacterium survives and multiplies within its host.
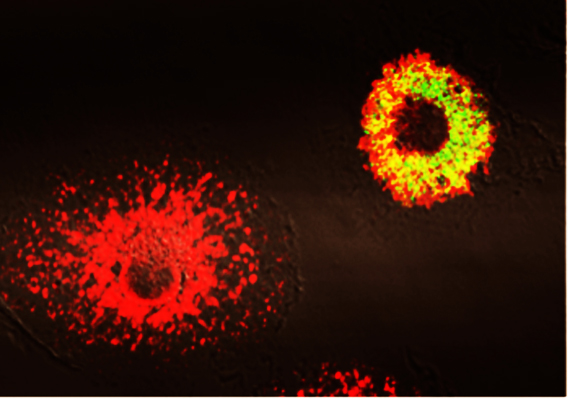
Phagocytes infected with Brucella abortus (in yellow). Copyright, JP Gorvel, CIML
To survive in the cell that is intended to eliminate it, Brucella, using constituents stolen from macrophages, produces a niche, called a vacuole, which protects it from the degradation machinery. Once the shelters are set up, then Brucella forms an infectious reservoir that is inaccessible to the immune system. "We have identified several molecules produced by Brucella to build this system of protection. These bacterial molecules are all potential therapeutic targets. Thus, Brucella strains that are genetically unable to produce them are efficiently degraded by macrophages," explains Jean-Pierre Gorvel.
"We also adapt the properties of these molecules to other purposes. Taken alone, some of them are able to increase the immune response against other infectious agents so they could be used as adjuvants for vaccines. We are currently testing this hypothesis in animal models in collaboration with the Institute of Biomedical Studies conducted by Jacques Banchereau at Baylor Hospital in Dallas."
From Brucella to tuberculosis, there is only one step, as Mycobacterium tuberculosis, the bacterium responsible for one of the most common infectious diseases in the world, hides, as does Brucella, inside macrophages. Thus, part of Jean-Pierre Gorvel's team, in collaboration with a group of researchers in South Africa, is interested in the virulence factors produced by M. tuberculosis.
In the lungs of infected patients, these molecules induce the formation of granulomas. Confinement of bacteria within these aggregates of tissues and immune cells allows them to protect themselves. They then enter a dormant state in accumulating energy reserves as lipids, which they obtain directly in macrophages. To escape the action of the immune system, M. tuberculosis synthesizes molecules distinct from those of Brucella. The team now uses electron microscopy to elucidate in detail the nature of interactions between macrophages and these Mycobacteria.
"We're trying to decipher the messages sent by Salmonella to host cells
but also to understand how these messages are interpreted."
Like its sister bacteria Brucella and Mycobacterium, Salmonella lives and multiplies within our cells. Of the 2000 family members now identified, the most famous are called Salmonella typhi, Salmonella paratyphi and Salmonella enteritidis. The first two are responsible for typhoid fever (21 million cases worldwide and nearly 200,000 deaths per year mainly in Africa, Asia and South America). These are diseases that can nevertheless be prevented by vaccination and the adoption of elementary hygiene aimed at preventing food contamination by feces of an infected person. The third lurks in food in vacuum packs in the bottom of our refrigerators. It is the cause of gastroenteritis affecting more than 2,000 French and 50,000 Americans each year.
"With the help of "micro-needles," Salmonella sends messages (in the form of proteins) into the host cell to modify the behavior of the latter," explains Stéphane Méresse, project manager within the team. "We try to decipher these messages but also to understand how the infected cell interprets them. Thus, we have shown that the bacterial proteins interact with human proteins to form complexes necessary for the development of Salmonella."
The story actually began several years ago at Imperial College in London where David Holden's team showed these bacterial proteins for the first time. In turn, the team cloned them in other bacteria and then re-expressed in association with a "label" which allows them to be visualized inside Salmonella and in infected human cells.
To identify human proteins to which they bind, the team used a screening technique called "double hybrid": the bacterial and human proteins are co-expressed in yeast cells that are genetically crippled but can restore their enzyme production system by using the bacterial / human protein for their metabolic needs. Thus, only surviving yeasts equipped with an effective complex are selected. The genes coding for the protein complex are then re-cloned and re-expressed in order to validate that the complex has been identically reconstructed.
"We found that in the absence of either of these proteins, the bacteria can't replicate or multiplies to levels so low that it is easily eliminated by the immune system," says Stéphane Méresse.
Are some molecules likely to block the formation of these complexes?" This is the question that the team of Jean Pierre Gorvel is now tackling. "With the help of experts in structural biology of the AFMB* we have established the crystal structure of one of these complexes. Now the objective is to identify a drug candidate capable of counteracting the organization to improve treatment of a disease that is resistant to more and more antibiotics."
Of immunology in human medicine to alimentary immunology
The team of Jean Pierre Gorvel also examines another aspect of immunity antibacterial, one that unfolds inside the digestive tract.
The gut contains a huge number of bacteria that the immune system must control to prevent them from passing into the bloodstream. These actors, local dendritic cells that are cousins of macrophages, reside in tissues and coordinate the involvement of other immune cells in response to infection. In the digestive tract, they sit in Peyer's patches, sentinel mucosal structures specifically dedicated to local immune surveillance.
"Within the Peyer's patches we have identified a novel class of dendritic cells, producing a potent antibacterial agent, lysozyme, identified in 1922 by Alexander Fleming, the famous co-discoverer of penicillin" says Jean-Pierre Gorvel. "This research is attracting much attention from the food processing industry. They are studying the action of probiotics in the gut. These microorganisms are used as food supplements in yogurt that exert a beneficial effect on consumer health. How do these Probiotics participate in intestinal immunity? Do they, for example, enable dendritic cells to become more effective in protecting consumers against infections?"
These different research areas illustrate the multitude of strategies developed by bacteria to survive attacks by our immune system, but also how the increased knowledge of these strategies are potentially applicable in various domains of human health.