The Laboratoire Hai-Tao He and Didier Marguet at the Centre d'Immunologie de Marseille-Luminy in partnership with Christophe Lamaze's research team from the Institut Curie and Céline Galès' team from the Institut des Maladies Métaboliques et Cardiovasculaires in Toulouse have jointly published an interdisciplinary study in Cell on August 11th 2016. This research decodes the dysfunction of molecular mechanisms involving interferon ɤ (IFNɤ), a key protein for immune defense, which is not able to fulfil its natural function as a protective cytokine when its receptor is "blocked" in the wrong place on the cell membrane: a modification to the immune response causing severe infections sometimes even life-threatening for young children.
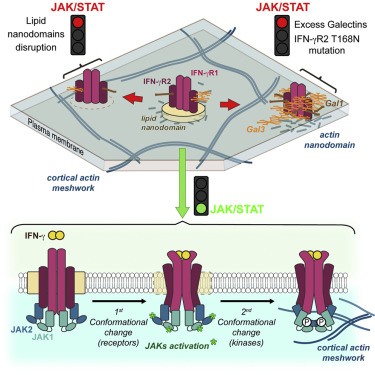 Interferon ɤ (IFNɤ) often described as the immune system's "star cytokine" is a protein produced in response to a virus, tumour or infectious attack. Like all interferons, IFNɤ is likely to trigger an immune response by attaching to a specific receptor present on the surface of some cells that in turn activates JAK/STAT signalling. Unlike other interferons, IFNɤ does not need to enter into the cell to fulfil its mission. However it has to be located in a specific area of the cell membrane with a diameter of only a few nanometres, in order to trigger a chain reaction leading to the stimulation of an immune response.
Interferon ɤ (IFNɤ) often described as the immune system's "star cytokine" is a protein produced in response to a virus, tumour or infectious attack. Like all interferons, IFNɤ is likely to trigger an immune response by attaching to a specific receptor present on the surface of some cells that in turn activates JAK/STAT signalling. Unlike other interferons, IFNɤ does not need to enter into the cell to fulfil its mission. However it has to be located in a specific area of the cell membrane with a diameter of only a few nanometres, in order to trigger a chain reaction leading to the stimulation of an immune response.
Hyperglycosylation of the IFNɤ receptor responsible for immune response failure
Christophe Lamaze, an INSERM research director at the Institut Curie, had already established the key role of IFNɤ in mendelian susceptibility to mycobacterial diseases (MSMD). The IFNɤ receptor is made up of two protein chains that normally have 6 sugars attached to them. However, some patients with this disease have 7 of them. It is therefore this simple glycolysation gain that disrupts all by itself the immune response to mycobacteria: "A seemingly unimportant modification to the IFNɤ receptor is responsible for this disease as the receptor is hyperglycosylated. Children affected by this genetic disease are susceptible to low virulence mycobacteria, such as Bacillus Calmette-Guérin (BCG) and environmental mycobacteria, and repeatedly suffer from serious infections" Christophe Lamaze explained.
Understanding a genetic disease by studying molecular movements
Hai-Tao He and Didier Marguet's team at the Centre d’Immunologie de Marseille-Luminy (Aix–Marseille University/Inserm/CNRS) brought their expertise in biophysics to this study. Their team has developed a spot variation Fluorescence Correlation Spectrocopy approach in order to study the diffusion mode of molecules on the cell surface. The result of analysis shows the existence of nanodomains (areas of the membrane only a few millionth of a millimetre in diameter) and demonstrates their fundamental role in signalling activity. "It was completely unexpected that the presence of only one additional sugar would be enough to modify how the IFNɤ receptor spreads across the cell surface, largely affecting the chain of events later on" Hai-Tao He explained. "Thus altered, the IFNɤ receptor is displaced to another type of nanodomain populated by galectin proteins that link to this additional sugar" added Yannick Hamon.
Considering galectins as a therapeutic target is an alluring prospect, particularly as switching off the expression of these proteins cancels in vitro the pathological effect linked to the glycolysation gain of the IFNɤ receptor. The researchers also note that mutations of this type represent up to 1.4% of mutations responsible for genetic diseases. However, they underline that the development of therapeutic programmes for these patients involves exploring and understanding the molecular mechanisms of the living in combination with the skills of biologists and doctors, as well as mathematicians and physicists, as experienced in this study.
SOURCE: http://www.cell.com/cell/fulltext/S0092-8674(16)30909-6
http://dx.doi.org/10.1016/j.cell.2016.07.003
VIDEO LINK: http://www.cell.com/cell/abstract/S0092-8674(16)30909-6?innerTabvideo-abstract_mmc3
AUTHORS: Cédric M Blouin, Yannick Hamon, Pauline Gonnord, Cédric Boularan, Jérémy Kagan, Christine Viaris de Lesegno, Richard Ruez, Sébastien Mailfert, Nicolas Bertaux, Damarys Loew, Christian Wunder, Ludger Johannes, Guillaume Vogt, Francesc-Xabier Contreras, Didier Marguet, Jean-Laurent Casanova, Céline Galès, Hai-Tao He, Christophe Lamaze.