We study interactions between immune components and developing tumors in a relevant microenvironment by using a mouse model of induced melanoma. These tumors express a known tumor antigen (TA). Requirements for differentiation of TA-specific CD8 T cells into cytolytic effectors capable of migrating into the tumors, of resisting the immunosuppressive tumor microenvironment and of efficiently killing tumors are studied at the molecular level and by fluorescence microscopy. Tumor development and T cell responses are also studied by in vivo imaging.
Overview
CD8 T Lymphocyte differentiation and function in the face of tumor development
T lymphocyte (TL) infiltration within primary tumors, their location and functional programs are essential parameters in determining clinical outcome. Thus, while a Th-1/CTL program involving expression of transcription factor T-bet and cytolytic enzymes such as granzyme B (Gzmb) correlated with a favorable prognosis in colorectal cancer (Galon et al., 2006), the basis for the polarization to this “Th-1” promoting rather than an inflammatory (IL1β, IL6, IL17) and immunosuppressive (TGFβ) tumor-promoting microenvironment is not clear. It may depend on an initial immune reaction to the tumor (DeNardo et al., 2009) or be associated with gene expression controlled by the oncogenic progression intrinsic to a given tumor type (Soudja et al., 2010). It is important to understand the molecular bases for imprinting of TL genetic programs and the extent to which effector or memory TL remain susceptible to modifications in the tumor microenvironment.
To this end, we are interested in establishing (i) the genetic marks imprinted by the context in which initial antigen (Ag)-dependent differentiation of CD8 TL occurs; (ii) whether CD8 TL may be stabilized for a given type of polarization and whether/how they may be reprogrammed.
An autochthonous melanoma model in mice
To address these questions in a relevant tumorigenesis context, we developed an autochthonous melanoma model in mice that recapitulates some aspects of human tumor development. These mice (TiRP mice) develop melanomas upon conditional deletion of the IInk4a/Arf tumor suppressor gene, with concomitant expression of oncogene H-RasG12V and a natural cancer-germline TA (Huijbers et al. 2006). Aggressively growing melanomas in TiRP mice are infiltrated by activated TL lacking Gzmb (Soudja et al. 2010). These cells express Programmed Death-1 (PD-1), an inhibitory receptor characteristic of chronically stimulated TL, as well as other receptors endowed with negative signaling properties (G. Verdeil, unpublished), also found on melanoma-infiltrating TL in patients. We sought to design an approach to modify intracellular components in CD8 TL to dampen negative signaling effects.
Sensitivity or resistance of CD8 anti-tumor T lymphocytes to mechanisms of immunosuppression: relevance for human and mouse inflammatory melanomas
The parameters that we are investigating in the context of anti-tumor CD8 TL responses are (i) induction and maintenance of effector molecules such as Gzmb; (ii) long term maintenance of proliferative capacity; (iii) capacity to migrate within tumors; (iv) resistance to the immunosuppressive tumor microenvironment.
We recently showed that CD8 effector TL expressing an active STAT5 transcription factor (STAT5CA), as a substitute for engagement of cytokine receptors sharing the γc chain (Verdeil et al. 2006), retain both long-term persistence and productive proliferation upon restimulation in addition to efficient effector functions (Grange et al. in preparation). These TL also appear to be functional in the immunosuppressive environment of aggressive melanomas.
We are further addressing by which intracellular mechanisms TL may become resistant to the effects of various inhibitory receptors such as PD-1, polarizing cytokines such as IL-6/STAT3 and TGFβ/Smad3 as well as amino-acid depleting enzymes such as arginase-1.
Whether the myeloid infiltrate and systemic cytokines observed in mice with aggressive melanomas (Soudja et al., 2010) are of prognostic value for a subset of melanoma patients is being further addressed (collaboration: JJ Grob, Hopital de la Timone, Marseille; D. Olive, IPC, Marseille).
Visualizing CD8 T Lymphocyte differentiation and function
Induction of Gzmb synthesis and localization of the enzyme in secretory granules is a hallmark of CD8 TL differentiation into effector TL capable of killing cognate target cells via granule exocytosis. Deficient TL effectors can result either from their suboptimal differentiation associated with poor synthesis of Gzmb, or from chronic antigenic stimulation leading to impaired TCR-dependent signaling.
A KI mouse expressing a Gzmb-tdTomato
To monitor the functional potential of CD8 TL, a KI mouse expressing a Gzmb-tdTomato construct in place of the Gzmb gene has been generated (P. Mouchacca and C. Boyer). In differentiated CD8 TL from these mice, Gzmb-tdTomato is found in Lamp1+ granules that are redirected to the TL-target contact zone where degranulation of fluorescent Gzmb-tdTomato occurs. This tool is used for analysis of TL differentiation and cytolytic activity in vivo during an immune response or tumor development, using fluorescence microscopy.
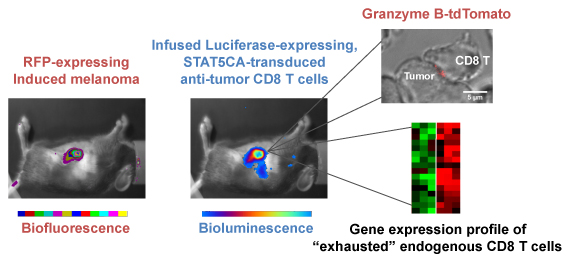
Monitoring melanoma development by biofluorescence and migration of anti-tumor CD8 T cells transduced with constitutive active STAT5 by bioluminescence. STAT5CA-transduced CD8 T cells express high level Gzmb in the tumor, whereas endogenous CD8 T cells have an exhausted phenotype with low Gzmb expression. CD8 T cells from a Gzmb-tdTomato KI mouse permit visualization of lytic granule polarization towards the tumor.